Vaccination With a Single Consensus Envelope Protein Ectodomain Sequence Administered in a Heterologous Regimen Induces Tetravalent Immune Responses and Protection Against Dengue Viruses in Mice
- PMID: 31134046
- PMCID: PMC6524413
- DOI: 10.3389/fmicb.2019.01113
Vaccination With a Single Consensus Envelope Protein Ectodomain Sequence Administered in a Heterologous Regimen Induces Tetravalent Immune Responses and Protection Against Dengue Viruses in Mice
Abstract
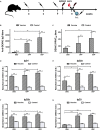
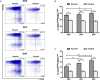
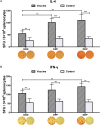
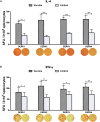
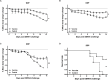
The development of a safe and effective tetravalent dengue vaccine that elicits protection against all dengue virus (DENV) serotypes is urgently needed. The consensus sequence of the ectodomain of envelope (E) protein of DENV (cE80) has been examined as an immunogen previously. In the current study, a cE80 DNA (D) vaccine was constructed and evaluated in conjunction with the cE80 protein (P) vaccine to examine whether both vaccines used together can further improve the immune responses. The cE80 DNA vaccine was administrated using either a homologous (DNA alone, DDD) or heterologous (DNA prime-protein boost: DDP or DPP) regimen, and evaluated for immunogenicity and protective efficacy in mice. Among the three DNA-based immunization regimens tested, DDP immunization is the optimal immunization regimen that elicited the greatest systemic immune response and conferred protection against all four DENV serotypes. This work provides innovative ideas for the development of consensus E-based dengue vaccines and the testing of optimal immunization regimens.
Keywords: E80; consensus sequence; dengue virus; envelope; prime-boost; tetravalent vaccine.
Figures

References
-
- Beltramello M., Williams K. L., Simmons C. P., Macagno A., Simonelli L., Quyen N. T., et al. (2010). The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8 271–283. 10.1016/j.chom.2010.08.007 - DOI - PMC - PubMed
-
- Cao W., Mishina M., Amoah S., Mboko W. P., Bohannon C., McCoy J., et al. (2018). Nasal delivery of H5N1 avian influenza vaccine formulated with GenJet or in vivo-jetPEI((R)) induces enhanced serological, cellular and protective immune responses. Drug Deliv. 25 773–779. 10.1080/10717544.2018.1450909 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

