FKN Facilitates HK-2 Cell EMT and Tubulointerstitial Lesions via the Wnt/β-Catenin Pathway in a Murine Model of Lupus Nephritis
- PMID: 31134047
- PMCID: PMC6524725
- DOI: 10.3389/fimmu.2019.00784
FKN Facilitates HK-2 Cell EMT and Tubulointerstitial Lesions via the Wnt/β-Catenin Pathway in a Murine Model of Lupus Nephritis
Abstract
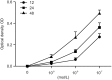
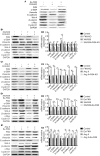
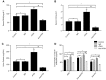
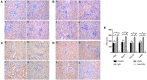
Fractalkine (FKN), also known as chemokine (C-X3-C motif) ligand 1, constitutes an intriguing chemokine with a documented role in the development of numerous inflammatory diseases including autoimmune disease. Specifically, it has been reported that FKN is involved in the disease progression of lupus nephritis (LN). The epithelial-mesenchymal transition (EMT) plays a significant role in the formation of tubulointerstitial lesions (TIL), which are increasingly recognized as a hallmark of tissue fibrogenesis after injury. However, the correlation between FKN and EMT or TIL in LN has not been determined. To investigate the potential role of FKN in EMT and TIL, MRL lymphoproliferation (MRL/lpr) strain mice were treated with an anti-FKN antibody, recombinant-FKN chemokine domain, or isotype antibody. Our results revealed that treatment with the anti-FKN antibody improved EMT, TIL, and renal function in MRL/lpr mice, along with inhibiting activation of the Wnt/β-catenin signaling pathway. In contrast, administration of the recombinant-FKN chemokine domain had the opposite effect. Furthermore, to further explore the roles of FKN in EMT, we assessed the levels of EMT markers in FKN-depleted or overexpressing human proximal tubule epithelial HK-2 cells. Our results provide the first evidence that the E-cadherin level was upregulated, whereas α-SMA and vimentin expression was downregulated in FKN-depleted HK-2 cells. In contrast, overexpression of FKN in HK-2 cells enhanced EMT. In addition, inhibition of the Wnt/β-catenin pathway by XAV939 negated the effect of FKN overexpression, whereas activation of the Wnt/β-catenin pathway by Ang II impaired the effect of the FKN knockout on EMT in HK-2 cells. Together, our data indicate that FKN plays essential roles in the EMT progression and development of TIL in MRL/lpr mice, most likely through activation of the Wnt/β-catenin signaling pathway.
Keywords: HK-2 cells; Wnt/β-catenin; epithelial-mesenchymal transition; fractalkine; murine model; tubulointerstitial lesion.
Figures


Similar articles
-
Fractalkine is Involved in Lipopolysaccharide-Induced Podocyte Injury through the Wnt/β-Catenin Pathway in an Acute Kidney Injury Mouse Model.Inflammation. 2019 Aug;42(4):1287-1300. doi: 10.1007/s10753-019-00988-1. Inflammation. 2019. PMID: 30919150 Free PMC article.
-
Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice.Arthritis Rheum. 2005 May;52(5):1522-33. doi: 10.1002/art.21007. Arthritis Rheum. 2005. PMID: 15880599
-
Fractalkine aggravates LPS-induced macrophage activation and acute kidney injury via Wnt/β-catenin signalling pathway.J Cell Mol Med. 2021 Jul;25(14):6963-6975. doi: 10.1111/jcmm.16707. Epub 2021 Jun 7. J Cell Mol Med. 2021. PMID: 34101346 Free PMC article.
-
A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy.Biomed Res Int. 2013;2013:987064. doi: 10.1155/2013/987064. Epub 2013 Dec 25. Biomed Res Int. 2013. PMID: 24455745 Free PMC article. Review.
-
WNT Signaling in Tumors: The Way to Evade Drugs and Immunity.Front Immunol. 2019 Dec 20;10:2854. doi: 10.3389/fimmu.2019.02854. eCollection 2019. Front Immunol. 2019. PMID: 31921125 Free PMC article. Review.
Cited by
-
Wnt signaling pathways in biology and disease: mechanisms and therapeutic advances.Signal Transduct Target Ther. 2025 Apr 4;10(1):106. doi: 10.1038/s41392-025-02142-w. Signal Transduct Target Ther. 2025. PMID: 40180907 Free PMC article. Review.
-
Efficacy of mesenchymal stem cells in treating tracheoesophageal fistula via the TLR4/NF-κb pathway in beagle macrophages.Heliyon. 2024 Jun 13;10(12):e32903. doi: 10.1016/j.heliyon.2024.e32903. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021940 Free PMC article.
-
Overexpression of hsa_circ_0061817 Can Inhibit the Proliferation and Invasion of Lung Cancer Cells Based on Active Compounds.Oxid Med Cell Longev. 2022 Sep 16;2022:4509019. doi: 10.1155/2022/4509019. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 39282154 Free PMC article.
-
Unraveling the proteomic landscape of fibrosis in lupus nephritis through CI-based analysis.Clin Rheumatol. 2024 Nov;43(11):3551-3564. doi: 10.1007/s10067-024-07140-x. Epub 2024 Sep 16. Clin Rheumatol. 2024. PMID: 39283550
-
Understanding the Renal Fibrotic Process in Leptospirosis.Int J Mol Sci. 2021 Oct 5;22(19):10779. doi: 10.3390/ijms221910779. Int J Mol Sci. 2021. PMID: 34639117 Free PMC article. Review.
References
-
- Bates MA, Akbari P, Gilley KN, Wagner JG, Li N, Kopec AK, et al. . Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol. (2018) 9:2002. 10.3389/fimmu.2018.02002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

