Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective
- PMID: 31134048
- PMCID: PMC6524697
- DOI: 10.3389/fimmu.2019.00789
Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective
Abstract
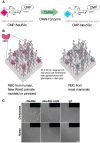
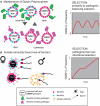
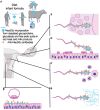
The glycocalyx of human cells differs from that of many other mammals by the lack of the sialic acid N-glycolylneuraminic acid (Neu5Gc) and increased abundance of its precursor N-acetylneuraminic acid (Neu5Ac). Most humans also have circulating antibodies specifically targeting the non-human sialic acid Neu5Gc. Recently, several additional mammalian species have been found to also lack Neu5Gc. In all cases, loss-of-function mutations in the gene encoding the sialic acid-modifying enzyme CMAH are responsible for the drastic change in these species. Unlike other glycan antigens, Neu5Gc apparently cannot be produced by microbes, raising the question about the origin of these antibodies in humans. Dietary exposure and presentation on bacteria coating themselves with Neu5Gc from the diet are distinct possibilities. However, the majority of the non-human species that lack Neu5Gc do not consume diets rich in Neu5Gc, making it unlikely that they will have been immunized against this sialic acid. A notable exception are mustelids (ferrets, martens and their relatives) known for preying on various small mammal species rich in Neu5Gc. No studies exist on levels of anti-Neu5Gc antibodies in non-human species. Evolutionary scenarios for the repeated, independent fixation of CMAH loss-of-function mutations at various time points in the past include strong selection by parasites, especially enveloped viruses, stochastic effects of genetic drift, and directional selection via female immunity to paternal Neu5Gc. Convergent evolution of losses of the vertebrate-specific self-glycan Neu5Gc are puzzling and may represent a prominent way in which glycans become agents of evolutionary change in their own right. Such change may include the reconfiguration of innate immune lectins that use self-sialic acids as recognition patterns.
Keywords: Neu5Ac; Neu5Gc; anti-glycan antibodies; sialic acid; xeno-antigen; xenoglycan.
Figures

References
-
- Varki A, Schnaar RL, Schauer R. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, et al. eds. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; (2015). p. 179–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

