Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) Among Patients With Antiphospholipid Antibodies (aPL)
- PMID: 31134052
- PMCID: PMC6514053
- DOI: 10.3389/fimmu.2019.00885
Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) Among Patients With Antiphospholipid Antibodies (aPL)
Abstract
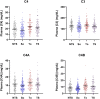
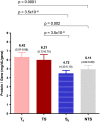
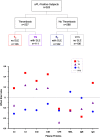
APS is the association of antiphospholipid antibodies (aPL) with thromboses and/or recurrent pregnancy loss (RPL). Among patients with SLE, one-third have aPL and 10-15% have a manifestation of secondary APS. Animal studies suggested that complement activation plays an important role in the pathogenesis of thrombosis and pregnancy loss in APS. We performed a cross-sectional study on complement proteins and genes in 525 patients with aPL. Among them, 237 experienced thromboses and 293 had SLE; 111 had both SLE and thromboses, and 106 had neither SLE nor thrombosis. Complement protein levels were determined by radial immunodiffusion for C4, C3 and factor H; and by functional ELISA for mannan binding lectin (MBL). Total C4, C4A and C4B gene copy numbers (GCN) were measured by TaqMan-based realtime PCR. Two to six copies of C4 genes are frequently present in a diploid genome, and each copy may code for an acidic C4A or a basic C4B protein. We observed significantly (a) higher protein levels of total C4, C4A, C4B, C3, and anticardiolipin (ACLA) IgG, (b) increased frequencies of lupus anticoagulant and males, and (c) decreased levels of complement factor H, MBL and ACLA-IgM among patients with thrombosis than those without thrombosis (N = 288). We also observed significantly lower GCNs of total C4 and C4A among aPL-positive patients with both SLE and thrombosis than others. By contrast, aPL-positive subjects with SLE had significantly reduced protein levels of C3, total C4, C4A, C4B and ACLA-IgG, and higher frequency of females than those without SLE. Patients with thrombosis but without SLE (N = 126), and patients with SLE but without thrombosis (N = 182) had the greatest differences in mean protein levels of C3 (p = 2.6 × 10-6), C4 (p = 2.2 × 10-9) and ACLA-IgG (p = 1.2 × 10-5). RPL occurred in 23.7% of female patients and thrombotic SLE patients had the highest frequency of RPL (41.0%; p = 3.8 × 10-10). Compared with non-RPL females, RPL had significantly higher frequency of thrombosis and elevated C4 protein levels. Female patients with homozygous C4A deficiency all experienced RPL (p = 0.0001) but the opposite was true for patients with homozygous C4B deficiency (p = 0.017). These results provide new insights and biomarkers for diagnosis and management of APS and SLE.
Keywords: C3 and C4; C4A and C4B; Copy number variation; Factor H; Lupus anticoagulant; Mannan binding lectin; Recurrent pregnancy loss; Thrombosis.
Figures
References
-
- Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. (1999) 42:1309–11. 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F - DOI - PubMed
-
- Schur PH. Pathogenesis of antiphospholipid syndrome. In: Pisetsky DS, ed. Up-To-Date. (2019). Available online at: https://www.uptodate.com/contents/pathogenesis-of-antiphospholipid-syndrome
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

