Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis
- PMID: 31134081
- PMCID: PMC6517498
- DOI: 10.3389/fimmu.2019.01011
Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis
Abstract
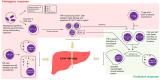
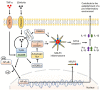
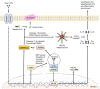
Human monocytic ehrlichiosis (HME) is a potentially life-threatening tick-borne rickettsial disease (TBRD) caused by the obligate intracellular Gram-negative bacteria, Ehrlichia. Fatal HME presents with acute ailments of sepsis and toxic shock-like symptoms that can evolve to multi-organ failure and death. Early clinical and laboratory diagnosis of HME are problematic due to non-specific flu-like symptoms and limitations in the current diagnostic testing. Several studies in murine models showed that cell-mediated immunity acts as a "double-edged sword" in fatal ehrlichiosis. Protective components are mainly formed by CD4 Th1 and NKT cells, in contrast to deleterious effects originated from neutrophils and TNF-α-producing CD8 T cells. Recent research has highlighted the central role of the inflammasome and autophagy as part of innate immune responses also leading to protective or pathogenic scenarios. Recognition of pathogen-associated molecular patterns (PAMPS) or damage-associated molecular patterns (DAMPS) triggers the assembly of the inflammasome complex that leads to multiple outcomes. Recognition of PAMPs or DAMPs by such complexes can result in activation of caspase-1 and -11, secretion of the pro-inflammatory cytokines IL-1β and IL-18 culminating into dysregulated inflammation, and inflammatory cell death known as pyroptosis. The precise functions of inflammasomes and autophagy remain unexplored in infections with obligate intracellular rickettsial pathogens, such as Ehrlichia. In this review, we discuss the intracellular innate immune surveillance in ehrlichiosis involving the regulation of inflammasome and autophagy, and how this response influences the innate and adaptive immune responses against Ehrlichia. Understanding such mechanisms would pave the way in research for novel diagnostic, preventative and therapeutic approaches against Ehrlichia and other rickettsial diseases.
Keywords: autophagy; ehrlichiosis; inflammasome; innate immunity; mechanism; pathogenesis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

