The IgA Isotype of Anti-β2 Glycoprotein I Antibodies Recognizes Epitopes in Domains 3, 4, and 5 That Are Located in a Lateral Zone of the Molecule (L-Shaped)
- PMID: 31134087
- PMCID: PMC6515947
- DOI: 10.3389/fimmu.2019.01031
The IgA Isotype of Anti-β2 Glycoprotein I Antibodies Recognizes Epitopes in Domains 3, 4, and 5 That Are Located in a Lateral Zone of the Molecule (L-Shaped)
Abstract
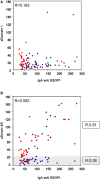
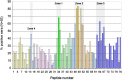
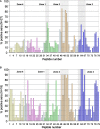
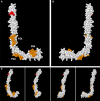
Background: Antiphospholipid syndrome (APS) is characterized by thrombosis and/or pregnancy morbidity with presence of anti-phospholipid antibodies (aPL). The APS classification criteria only consider the aPL of IgG/IgM isotype, however testing of aPL of IgA isotype is recommended when APS is suspected and consensus aPL are negative. IgA anti-βeta-2 glycoprotein-I (B2GP1) has been clearly related with occurrence of thrombotic events. Antibodies anti-B2GP1 of IgG/M isotypes recognize an epitope in Domain 1 (R39-G43), the epitopes that recognize IgA anti-B2GP1 antibodies are not well-identified. Aim: To determine the zones of B2GP1 recognized by antibodies of IgA isotype from patients with APS symptomatology and positive for IgA anti-B2GP1. Methods: IgA antibodies to Domain-1(D1) and Domain-4/5(D4/5) of B2GP1 (ELISA) and epitope mapping on oligopeptide arrays of B2GP1 were evaluated in sera from a group of 93 patients with at least one thrombotic and with isolated positivity for IgA anti-B2GP1 antibodies (negative for other aPL). Results: A total of 47 patients (50.5%) were positive for anti-D4/5 and 23(25%) were positive for anti-D1. When peptide arrays were analyzed, three zones of B2GP1 reactivity were identified for more than 50% of patients. The center of these zones corresponds to amino acids 140(D3), 204(D4), and 264(D5). The peptides recognized on D3 and D4 contain amino acid sequences sharing high homology with proteins of microorganism that were previously related with a possible APS infectious etiology. In the three-dimensional structure of B2GP1, the three peptides, as the R39-G43 epitope, are located on the right side of the molecule (L-shape). The left side (J-shape) does not bind the antibodies. Conclusions: Patients with thrombotic APS clinical-criteria, and isolated IgA anti-B2GP1 positivity appear to preferentially bind, not to the D1 or D4/5 domains of B2GP1, but rather to three sites in D3, D4, and D5. The sites on D3 and D4 were previously described as the target identified by human monoclonal antibodies derived from patients that were capable of inducing APS in animal models. The localization of these epitopes opens a new route to explore to increase understanding of the patholophysiology of the APS and to propose new alternatives and therapeutic targets.
Keywords: B2GP1; antiphospholipid antibodies; antiphospholipid syndrome; epitope mapping; graft thrombosis; kidney transplant; peptide arrays.
Figures

Similar articles
-
Clinical characterization of antiphospholipid syndrome by detection of IgG antibodies against β2 -glycoprotein i domain 1 and domain 4/5: ratio of anti-domain 1 to anti-domain 4/5 as a useful new biomarker for antiphospholipid syndrome.Arthritis Rheumatol. 2015 May;67(8):2196-204. doi: 10.1002/art.39187. Arthritis Rheumatol. 2015. PMID: 25939498
-
Measuring IgA Anti-β2-Glycoprotein I and IgG/IgA Anti-Domain I Antibodies Adds Value to Current Serological Assays for the Antiphospholipid Syndrome.PLoS One. 2016 Jun 2;11(6):e0156407. doi: 10.1371/journal.pone.0156407. eCollection 2016. PLoS One. 2016. PMID: 27253369 Free PMC article.
-
IgG/IgM antiphospholipid antibodies present in the classification criteria for the antiphospholipid syndrome: a critical review of their association with thrombosis.J Thromb Haemost. 2016 Aug;14(8):1530-48. doi: 10.1111/jth.13379. Epub 2016 Aug 11. J Thromb Haemost. 2016. PMID: 27279342 Review.
-
Immunoglobulin A Isotype of Antiphospholipid Antibodies Does Not Provide Added Value for the Diagnosis of Antiphospholipid Syndrome in a Chinese Population.Front Immunol. 2020 Oct 5;11:568503. doi: 10.3389/fimmu.2020.568503. eCollection 2020. Front Immunol. 2020. PMID: 33123140 Free PMC article.
-
Noncriteria antiphospholipid antibodies in antiphospholipid syndrome.Int J Lab Hematol. 2024 May;46 Suppl 1:34-42. doi: 10.1111/ijlh.14268. Epub 2024 Apr 7. Int J Lab Hematol. 2024. PMID: 38584293 Review.
Cited by
-
Molecular Mechanisms of "Antiphospholipid Antibodies" and Their Paradoxical Role in the Pathogenesis of "Seronegative APS".Int J Mol Sci. 2020 Nov 9;21(21):8411. doi: 10.3390/ijms21218411. Int J Mol Sci. 2020. PMID: 33182499 Free PMC article. Review.
-
Current Promising Biomarkers and Methods in the Diagnostics of Antiphospholipid Syndrome: A Review.Biomedicines. 2021 Feb 8;9(2):166. doi: 10.3390/biomedicines9020166. Biomedicines. 2021. PMID: 33567576 Free PMC article. Review.
-
Ischemic stroke in anti-β2-glycoprotein I IgA-associated non-criteria antiphospholipid syndrome: a case report of arterial recanalization via antiplatelet therapy.Front Immunol. 2025 Jul 4;16:1603526. doi: 10.3389/fimmu.2025.1603526. eCollection 2025. Front Immunol. 2025. PMID: 40688083 Free PMC article.
-
High IgA antiphospholipid autoantibodies in healthy Sudanese explain the increased prevalence among Sudanese compared to Swedish systemic lupus erythematosus patients.Lupus. 2020 Oct;29(11):1412-1422. doi: 10.1177/0961203320945387. Epub 2020 Aug 2. Lupus. 2020. PMID: 32741301 Free PMC article.
-
Elevated IgA antiphospholipid antibodies in healthy pregnant women in Sudan but not Sweden, without corresponding increase in IgA anti-β2 glycoprotein I domain 1 antibodies.Lupus. 2020 Apr;29(5):463-473. doi: 10.1177/0961203320908949. Epub 2020 Feb 27. Lupus. 2020. PMID: 32106789 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

