Effect of ethnicity on vinorelbine pharmacokinetics: a population pharmacokinetics analysis
- PMID: 31134323
- PMCID: PMC6647192
- DOI: 10.1007/s00280-019-03872-9
Effect of ethnicity on vinorelbine pharmacokinetics: a population pharmacokinetics analysis
Abstract
Background: Pharmacokinetics of vinorelbine is mainly known from studies conducted in European patients. Interethnic differences in drug disposition may, however, induce interethnic variation in drug exposure. This paper aimed to evaluate the effect of ethnicity on the bioavailability and clearance of oral and intravenous vinorelbine.
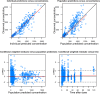
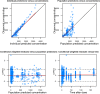
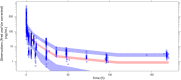
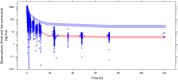
Methods: Oral and intravenous vinorelbine pharmacokinetics data in Asian patients were pooled from two-phase II studies of patients with non-small-cell lung cancer or advanced breast cancer in China. Blood vinorelbine and its active metabolite, 4'-O-deacetylvinorelbine, were quantified using liquid chromatography-tandem mass spectrometry. Bayesian pharmacokinetic parameters were calculated and vinorelbine monotherapy results (intravenous 25 mg/m2; oral 60 mg/m2) of the Asian data set were compared to a reference European data set (intravenous 30 mg/m2; oral 80 mg/m2). Subsequently, a population pharmacokinetics analysis was conducted in a combined cohort (Asian data set + historical vinorelbine pharmacokinetics database) to investigate for a potential effect of ethnicity.
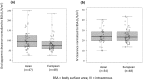
Results: Pharmacokinetics data from the Asian data set (oral: n = 47; intravenous: n = 34) was compared to the European reference data set (oral: n = 48; intravenous: n = 48). Mean apparent clearance of oral vinorelbine and mean absolute clearance of intravenous vinorelbine was comparable between the Asian and reference European data set. A population pharmacokinetic analysis (oral: n = 222; intravenous: n = 111) demonstrated no influence of ethnicity on oral and intravenous vinorelbine bioavailability and clearance.
Conclusion: Vinorelbine pharmacokinetics were found to be comparable between Asian and European patients. No relevant influence of ethnicity on vinorelbine bioavailability and clearance for oral and intravenous routes of administration was observed.
Keywords: Breast cancer; China; Non-small-cell lung cancer; Pharmacokinetics; Vinorelbine.
Conflict of interest statement
AP, GZ, and PF are employees of Pierre Fabre Research Institute. All other authors have no conflict of interest to disclose.
Figures
References
-
- Pierre Fabre Press Release (2014, Sept 24) Pierre Fabre Médicament obtains MA in China for NAVELBINE® Oral (Vinorelbine) in the treatment of advanced lung and breast cancer. https://www.pierre-fabre.com/en/news/pierre-fabre-medicament-obtains-ma-...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

