Blockade of Acid-Sensing Ion Channels Attenuates Recurrent Hypoglycemia-Induced Potentiation of Ischemic Brain Damage in Treated Diabetic Rats
- PMID: 31134484
- PMCID: PMC8918033
- DOI: 10.1007/s12017-019-08546-6
Blockade of Acid-Sensing Ion Channels Attenuates Recurrent Hypoglycemia-Induced Potentiation of Ischemic Brain Damage in Treated Diabetic Rats
Abstract
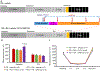
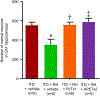
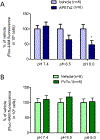
Diabetes is a chronic metabolic disease and cerebral ischemia is a serious complication of diabetes. Anti-diabetic therapy mitigates this complication but increases the risk of exposure to recurrent hypoglycemia (RH). We showed previously that RH exposure increases ischemic brain damage in insulin-treated diabetic (ITD) rats. The present study evaluated the hypothesis that increased intra-ischemic acidosis in RH-exposed ITD rats leads to pronounced post-ischemic hypoperfusion via activation of acid-sensing (proton-gated) ion channels (ASICs). Streptozotocin-diabetic rats treated with insulin were considered ITD rats. ITD rats were exposed to RH for 5 days and were randomized into Psalmotoxin1 (PcTx1, ASIC1a inhibitor), APETx2 (ASIC3 inhibitor), or vehicle groups. Transient global cerebral ischemia was induced overnight after RH. Cerebral blood flow was measured using laser Doppler flowmetry. Ischemic brain injury in hippocampus was evaluated using histopathology. Post-ischemic hypoperfusion in RH-exposed rats was of greater extent than that in control rats. Inhibition of ASICs prevented RH-induced increase in the extent of post-ischemic hypoperfusion and ischemic brain injury. Since ASIC activation-induced store-operated calcium entry (SOCE) plays a role in vascular tone, next we tested if acidosis activates SOCE via activating ASICs in vascular smooth muscle cells (VSMCs). We observed that SOCE in VSMCs at lower pH is ASIC3 dependent. The results show the role of ASIC in post-ischemic hypoperfusion and increased ischemic damage in RH-exposed ITD rats. Understanding the pathways mediating exacerbated ischemic brain injury in RH-exposed ITD rats may help lower diabetic aggravation of ischemic brain damage.
Keywords: APETx2; Acidosis; Cerebral blood flow; Psalmotoxin1; Store-operated calcium entry; Vascular smooth muscle cells.
Conflict of interest statement
Conflicts of interest:
The authors declare that they have no conflict of interest.
Figures
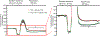
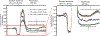
References
-
- Almdal T, Scharling H, Jensen JS, & Vestergaard H (2004). The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med, 164(13), 1422–1426, doi: 10.1001/archinte.164.13.1422. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

