Mechanochemistry in Translation
- PMID: 31134795
- PMCID: PMC6879781
- DOI: 10.1021/acs.biochem.9b00260
Mechanochemistry in Translation
Abstract
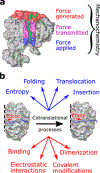
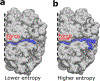
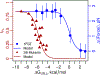
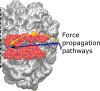
As the influence of translation rates on protein folding and function has come to light, the mechanisms by which translation speed is modulated have become an important issue. One mechanism entails the generation of force by the nascent protein. Cotranslational processes, such as nascent protein folding, the emergence of unfolded nascent chain segments from the ribosome's exit tunnel, and insertion of the nascent chain into or translocation of the nascent chain through membranes, can generate forces that are transmitted back to the peptidyl transferase center and affect translation rates. In this Perspective, we examine the processes that generate these forces, the mechanisms of transmission along the ribosomal exit tunnel to the peptidyl transferase center, and the effects of force on the ribosome's catalytic cycle. We also discuss the physical models that have been developed to predict and explain force generation for individual processes and speculate about other processes that may generate forces that have yet to be tested.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Takacs L (2013) The historical development of mechano-chemistry. Chem. Soc. Rev 42, 7649–7659. - PubMed
-
- Wang GW, Komatsu K, Murata Y, and Shiro M (1997) Synthesis and X-ray structure of dumb-bell-shaped C120. Nature 387, 583–586.
-
- Komatsu K, Wang GW, Murata Y, Tanaka T, Fujiwara K, Yamamoto K, and Saunders M (1998) Mechanochemical Synthesis and Characterization of the Fullerene Dimer C120. J. Org. Chem 63, 9358–9366.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

