Late-stage functionalization of BN-heterocycles
- PMID: 31134984
- PMCID: PMC6609152
- DOI: 10.1039/c9cs00218a
Late-stage functionalization of BN-heterocycles
Abstract
BN/CC isosterism has emerged as a viable strategy to expand the chemical space of organic molecules. In particular, the application of BN/CC isosterism to arenes has received significant attention due to the vast available chemical space provided by aromatic hydrocarbons. The synthetic efforts directed at assembling novel aromatic BN heterocycles have resulted in the discovery of new properties and functions in a variety of fields including biomedical research, medicinal chemistry, materials science, catalysis, and organic synthesis. This tutorial review specifically covers recent advances in synthetic technologies that functionalize assembled boron-nitrogen (BN) heterocycles and highlights their distinct reactivity and selectivity in comparison to their carbonaceous counterparts. It is intended to serve as a state-of-the-art compendium for readers who are interested in the reaction chemistry of BN heterocycles.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
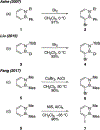
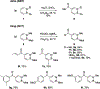
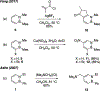
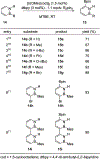
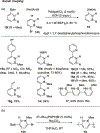
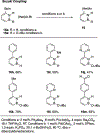

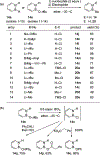

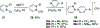
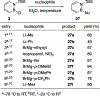

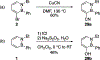
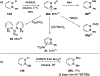
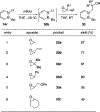
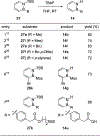
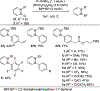


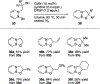
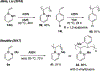
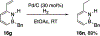

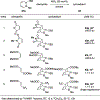
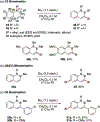
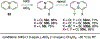
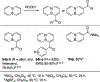
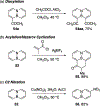
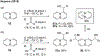
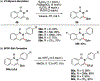

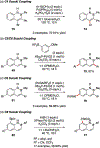
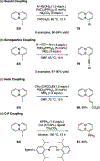
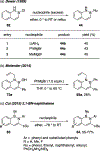

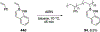
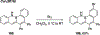
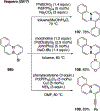
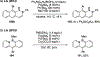
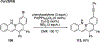
References
-
- For an overview of BN/CC isosterism in aromatic compounds, see: Bosdet MJD and Piers WE, Can. J. Chem, 2009, 87, 8–29.
-
- For recent developments in azaborine chemistry, see: Bélanger-Chabot G, Braunschweig H and Roy DK, Eur. J. Inorg. Chem, 2017, (38–39), 4353–4368.
-
- Pan J, Kampf JW and Ashe AJ, Org. Lett, 2007, 9, 679–681. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

