Inhibiting Autophagy in Renal Cell Cancer and the Associated Tumor Endothelium
- PMID: 31135523
- PMCID: PMC10395074
- DOI: 10.1097/PPO.0000000000000374
Inhibiting Autophagy in Renal Cell Cancer and the Associated Tumor Endothelium
Abstract
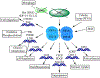
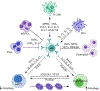
The clear cell subtype of kidney cancer encompasses most renal cell carcinoma cases and is associated with the loss of von Hippel-Lindau gene function or expression. Subsequent loss or mutation of the other allele influences cellular stress responses involving nutrient and hypoxia sensing. Autophagy is an important regulatory process promoting the disposal of unnecessary or degraded cellular components, tightly linked to almost all cellular processes. Organelles and proteins that become damaged or that are no longer needed in the cell are sequestered and digested in autophagosomes upon fusing with lysosomes, or alternatively, released via vesicular exocytosis. Tumor development tends to disrupt the regulation of the balance between this process and apoptosis, permitting prolonged cell survival and increased replication. Completed trials of autophagic inhibitors using hydroxychloroquine in combination with other anticancer agents including rapalogues and high-dose interleukin 2 have now been reported. The complex nature of autophagy and the unique biology of clear cell renal cell carcinoma warrant further understanding to better develop the next generation of relevant anticancer agents.
Figures
Similar articles
-
MicroRNAs Associated with Von Hippel-Lindau Pathway in Renal Cell Carcinoma: A Comprehensive Review.Int J Mol Sci. 2017 Nov 22;18(11):2495. doi: 10.3390/ijms18112495. Int J Mol Sci. 2017. PMID: 29165391 Free PMC article. Review.
-
Inhibiting autophagy: a novel approach for the treatment of renal cell carcinoma.Cancer J. 2013 Jul-Aug;19(4):341-7. doi: 10.1097/PPO.0b013e31829da0d6. Cancer J. 2013. PMID: 23867516
-
The emerging role of nuclear factor kappa B in renal cell carcinoma.Int J Biochem Cell Biol. 2011 Nov;43(11):1537-49. doi: 10.1016/j.biocel.2011.08.003. Epub 2011 Aug 12. Int J Biochem Cell Biol. 2011. PMID: 21854869 Review.
-
Action of YM155 on clear cell renal cell carcinoma does not depend on survivin expression levels.PLoS One. 2017 Jun 5;12(6):e0178168. doi: 10.1371/journal.pone.0178168. eCollection 2017. PLoS One. 2017. PMID: 28582447 Free PMC article.
-
Gramicidin A induces metabolic dysfunction and energy depletion leading to cell death in renal cell carcinoma cells.Mol Cancer Ther. 2013 Nov;12(11):2296-307. doi: 10.1158/1535-7163.MCT-13-0445. Epub 2013 Sep 4. Mol Cancer Ther. 2013. PMID: 24006494
Cited by
-
Identification of autophagy-related biomarker and analysis of immune infiltrates in oral carcinoma.J Clin Lab Anal. 2022 May;36(5):e24417. doi: 10.1002/jcla.24417. Epub 2022 Apr 14. J Clin Lab Anal. 2022. PMID: 35421271 Free PMC article.
-
An actin-WHAMM interaction linking SETD2 and autophagy.Biochem Biophys Res Commun. 2021 Jun 18;558:202-208. doi: 10.1016/j.bbrc.2020.09.025. Epub 2020 Oct 6. Biochem Biophys Res Commun. 2021. PMID: 33036756 Free PMC article.
-
SETD2 mutation in renal clear cell carcinoma suppress autophagy via regulation of ATG12.Cell Death Dis. 2020 Jan 27;11(1):69. doi: 10.1038/s41419-020-2266-x. Cell Death Dis. 2020. PMID: 31988284 Free PMC article.
-
Construction autophagy-related prognostic risk signature combined with clinicopathological validation analysis for survival prediction of kidney renal papillary cell carcinoma patients.BMC Cancer. 2021 Apr 15;21(1):411. doi: 10.1186/s12885-021-08139-2. BMC Cancer. 2021. PMID: 33858375 Free PMC article.
-
Comprehensive analysis of autophagy associated genes and immune infiltrates in cervical cancer.Iran J Basic Med Sci. 2024;27(7):813-824. doi: 10.22038/IJBMS.2024.74431.16168. Iran J Basic Med Sci. 2024. PMID: 38800011 Free PMC article.
References
-
- Lotze MT, Maranchie J, and Appleman L, Inhibiting autophagy: a novel approach for the treatment of renal cell carcinoma. Cancer J, 2013. 19(4): p. 341–7. - PubMed
-
- Eckardt KU, et al., Hypoxia-inducible transcription factors and their role in renal disease. Semin Nephrol, 2007. 27(3): p. 363–72. - PubMed
-
- Bouhamdani N, et al., Quantitative proteomics to study a small molecule targeting the loss of von Hippel-Lindau in renal cell carcinomas. Int J Cancer, 2017. 141(4): p. 778–790. - PubMed
-
- Maranchie JK, et al., The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell, 2002. 1(3): p. 247–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

