An Overview of Cancer Drugs Approved by the US Food and Drug Administration Based on the Surrogate End Point of Response Rate
- PMID: 31135822
- PMCID: PMC6547222
- DOI: 10.1001/jamainternmed.2019.0583
An Overview of Cancer Drugs Approved by the US Food and Drug Administration Based on the Surrogate End Point of Response Rate
Abstract
Importance: Approximately one-third of cancer drugs are approved based on response rate (RR)-the percentage of patients whose tumors shrink beyond an arbitrary threshold-typically assessed in a single-arm study.
Objective: To characterize RR end points used by the US Food and Drug Administration (FDA) for cancer drug approval.
Design, setting, and participants: A retrospective review of FDA-approved drug indications in oncology from 2006 to 2018.
Exposures: Data related to cancer type, line of therapy (first-line, second-line, or third-or-later-line treatment for advanced/metastatic disease), type of FDA approval pathway, trial design, sample size, and level of innovation were extracted.
Main outcomes and measures: The primary outcome was the RR used as the basis for FDA approval. The secondary outcome was rate of complete response.
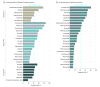
Results: Eighty-five indications for 59 cancer drugs were identified, 32 (38%) received regular approval, and 53 (62%) were granted accelerated approval. Twenty-nine (55%) accelerated approvals were later converted to regular approval. Of these, 6 (21%) approvals showed overall survival benefit, 16 (55%) later established progression-free survival benefit, and 7 (24%) continued to use RR but gained regular approval. The median RR among the 85 indications was 41% (interquartile range [IQR], 27%-58%). Among them, 14 of 85 (16%) had an RR less than 20%, 28 of 85 (33%) had an RR less than 30%, and 40 of 85 (47%) had an RR less than 40%. The median complete RR for 81 participants was 6% (IQR, 2%-22%). The median sample size among studies leading to approval was 117 (IQR, 76-182; range, 18-1052 participants). Drugs with accelerated approval pending confirmatory data had lower RR compared with drugs that have completed most postmarketing efficacy requirements (median, 28%; IQR, 15%-50% vs median, 42%; IQR, 31%-58%; P = .02).
Conclusions and relevance: Many cancer drugs approved on the basis of response rate offer numerically low or modest response rates. Most premarket studies accrue more than 100 patients. Some of these drugs could potentially be tested in premarket randomized clinical trials measuring directly end points that demonstrate clinical benefit.
Conflict of interest statement
Figures

Comment in
-
Accelerated Approval of Cancer Drugs-Righting the Ship of the US Food and Drug Administration.JAMA Intern Med. 2019 Jul 1;179(7):922-923. doi: 10.1001/jamainternmed.2019.0584. JAMA Intern Med. 2019. PMID: 31135826 No abstract available.
-
An International Perspective on Drugs for Cancer: The Best of Times, the Worst of Times.JAMA Intern Med. 2019 Jul 1;179(7):913-914. doi: 10.1001/jamainternmed.2019.0458. JAMA Intern Med. 2019. PMID: 31135827 No abstract available.
References
-
- Blumenthal GM, Karuri SW, Zhang H, et al. . Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33(9):1008-1014. doi:10.1200/JCO.2014.59.0489 - DOI - PMC - PubMed

