Long-term Outcomes in a Large Randomized Trial of HIV-1 Salvage Therapy: 96-Week Results of AIDS Clinical Trials Group A5241 (OPTIONS)
- PMID: 31135883
- PMCID: PMC7137888
- DOI: 10.1093/infdis/jiz281
Long-term Outcomes in a Large Randomized Trial of HIV-1 Salvage Therapy: 96-Week Results of AIDS Clinical Trials Group A5241 (OPTIONS)
Abstract
Background: Short-term (48-week) results of the OPTIONS trial showed that nucleoside reverse transcriptase inhibitors (NRTIs) can be safely omitted from salvage therapy as long as the regimen has a cumulative activity of >2 active antiretroviral medications. The long-term durability of this approach and outcomes in persons who have more-extensive HIV-1 drug resistance are uncertain.
Methods: Participants with virologic failure and anticipated antiretroviral susceptibility received an optimized regimen and were randomized to omit or add NRTIs. A separate group with more resistance (cumulative activity ≤2 active agents) received an optimized regimen including NRTIs.
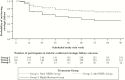
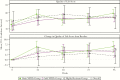
Results: At week 96, among 360 participants randomized to omit or add NRTIs, 70% and 65% had HIV-1 RNA <200 copies/mL, respectively. Virologic failure was uncommon after week 48. Younger age and starting fewer new antiretroviral medications were associated with higher odds of virologic failure. In the highly resistant group, 53% had HIV-1 RNA <200 copies/mL at week 96.
Conclusions: HIV-1 salvage therapy can safely omit NRTIs without compromising efficacy or durability of response as long as the new regimen has a cumulative activity of >2 active drugs. Younger people and those receiving fewer new antiretrovirals require careful monitoring. Even among individuals with more-extensive resistance, most achieve virologic suppression.
Clinical trials registration: NCT00537394.
Keywords: HIV-1; antiretroviral therapy; drug resistance; randomized controlled trial; salvage therapy; treatment-experienced participants.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Salvage Antiretroviral Therapy: Time for "DeNUKElearization"?J Infect Dis. 2020 Apr 7;221(9):1390-1393. doi: 10.1093/infdis/jiz283. J Infect Dis. 2020. PMID: 31136663 Free PMC article. No abstract available.
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Department of Health and Human Services; https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/v.... Accessed 24 December 2017.
-
- Stanford University. HIV drug resistance database https://hivdb.stanford.edu/. Accessed 5 June 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

