Induction of brain-infiltrating T-bet-expressing B cells in multiple sclerosis
- PMID: 31136008
- PMCID: PMC6771938
- DOI: 10.1002/ana.25508
Induction of brain-infiltrating T-bet-expressing B cells in multiple sclerosis
Abstract
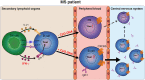
Objective: Results from anti-CD20 therapies demonstrate that B- and T-cell interaction is a major driver of multiple sclerosis (MS). The local presence of B-cell follicle-like structures and oligoclonal bands in MS patients indicates that certain B cells infiltrate the central nervous system (CNS) to mediate pathology. Which peripheral triggers underlie the development of CNS-infiltrating B cells is not fully understood.
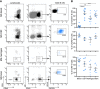
Methods: Ex vivo flow cytometry was used to assess chemokine receptor profiles of B cells in blood, cerebrospinal fluid, meningeal, and brain tissues of MS patients (n = 10). Similar analyses were performed for distinct memory subsets in the blood of untreated and natalizumab-treated MS patients (n = 38). To assess T-bet(CXCR3)+ B-cell differentiation, we cultured B cells from MS patients (n = 21) and healthy individuals (n = 34) under T helper 1- and TLR9-inducing conditions. Their CNS transmigration capacity was confirmed using brain endothelial monolayers.
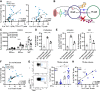
Results: CXC chemokine receptor 3 (CXCR3)-expressing B cells were enriched in different CNS compartments of MS patients. Treatment with the clinically effective drug natalizumab prevented the recruitment of CXCR3high IgG1+ subsets, corresponding to their increased ability to cross CNS barriers in vitro. Blocking of interferon-γ (IFNγ) reduced the transmigration potential and antigen-presenting function of these cells. IFNγ-induced B cells from MS patients showed increased T-bet expression and plasmablast development. Additional TLR9 triggering further upregulated T-bet and CXCR3, and was essential for IgG1 switching.
Interpretation: This study demonstrates that T-bethigh IgG1+ B cells are triggered by IFNγ and TLR9 signals, likely contributing to enhanced CXCR3-mediated recruitment and local reactivity in the CNS of MS patients. ANN NEUROL 2019;86:264-278.
© 2019 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

