Involvement of rat posterior prelimbic and cingulate area 2 in vocalization control
- PMID: 31136026
- PMCID: PMC6899747
- DOI: 10.1111/ejn.14477
Involvement of rat posterior prelimbic and cingulate area 2 in vocalization control
Abstract
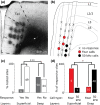
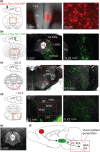
Microstimulation mapping identified vocalization areas in primate anterior cingulate cortex. Rat anterior cingulate and medial prefrontal areas have also been intensely investigated, but we do not know, how these cortical areas contribute to vocalizations and no systematic mapping of stimulation-evoked vocalizations has been performed. To address this question, we mapped microstimulation-evoked (ultrasonic) vocalizations in rat cingulate and medial prefrontal cortex. The incidence of evoked vocalizations differed markedly between frontal cortical areas. Vocalizations were most often evoked in posterior prelimbic cortex and cingulate area 2, whereas vocalizations were rarely evoked in dorsal areas (vibrissa motor cortex, secondary motor cortex and cingulate area 1) and anterior areas (anterior prelimbic, medial-/ventral-orbital cortex). Vocalizations were observed at intermediate frequencies in ventro-medial areas (infralimbic and dorsopeduncular cortex). Various complete, naturally occurring calls could be elicited. In prelimbic cortex superficial layer microstimulation evoked mainly fear calls with low efficacy, whereas deep layer microstimulation evoked mainly 50 kHz calls with high efficacy. Vocalization stimulation thresholds were substantial (70-500 μA, the maximum tested; on average ~400 μA) and latencies were long (median 175 ms). Posterior prelimbic cortex projected to numerous targets and innervated brainstem vocalization centers such as the intermediate reticular formation and the nucleus retroambiguus disynaptically via the periaqueductal gray. Anatomical position, stimulation effects and projection targets of posterior prelimbic cortex were similar to that of monkey anterior cingulate vocalization cortex. Our data suggest that posterior prelimbic cortex is more closely involved in control of vocalization initiation than in specifying acoustic details of vocalizations.
Keywords: USVs; microstimulation; periaqueductal gray; prefrontal cortex; transsynaptic tracing.
© 2019 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Aitken, P. G. (1981). Cortical control of conditioned and spontaneous vocal behavior in rhesus monkeys. Brain and Language, 13(1), 171–184. - PubMed
-
- Brecht, M. , Krauss, A. , Muhammad, S. , Sinai‐Esfahani, L. , Bellanca, S. , & Margrie, T. W. (2004). Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. The Journal of Comparative Neurology, 479(4), 360–373. - PubMed
-
- Burgdorf, J. , Wood, P. L. , Kroes, R. A. , Moskal, J. R. , & Panksepp, J. (2007). Neurobiology of 50‐kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behavioral Brain Research, 182(2), 274–283. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

