Termination of bleeding by a specific, anticatalytic antibody against plasmin
- PMID: 31136076
- PMCID: PMC7359864
- DOI: 10.1111/jth.14522
Termination of bleeding by a specific, anticatalytic antibody against plasmin
Abstract
Background: Excessive, plasmin-mediated fibrinolysis augments bleeding and contributes to death in some patients. Current therapies for fibrinolytic bleeding are limited by modest efficacy, low potency, and off-target effects.
Objectives: To determine whether an antibody directed against unique loop structures of the plasmin protease domain may have enhanced specificity and potency for blocking plasmin activity, fibrinolysis, and experimental hemorrhage.
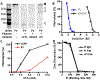
Methods: The binding specificity, affinity, protease cross-reactivity and antifibrinolytic properties of a monoclonal plasmin inhibitor antibody (Pi) were examined and compared with those of epsilon aminocaproic acid (EACA), which is a clinically used fibrinolysis inhibitor.
Results: Pi specifically recognized loop 5 of the protease domain, and did not bind to other serine proteases or nine other non-primate plasminogens. Pi was ~7 logs more potent in neutralizing plasmin cleavage of small-molecule substrates and >3 logs more potent in quenching fibrinolysis than EACA. Pi was similarly effective in blocking catalysis of a small-molecule substrate as α2 -antiplasmin, which is the most potent covalent inhibitor of plasmin, and was a more potent fibrinolysis inhibitor. Fab or chimerized Fab fragments of Pi were equivalently effective. In vivo, in a humanized model of fibrinolytic surgical bleeding, Pi significantly reduced bleeding to a greater extent than a clinical dose of EACA.
Conclusions: A mAb directed against unique loop sequences in the protease domain is a highly specific, potent, competitive plasmin inhibitor that significantly reduces experimental surgical bleeding in vivo.
Keywords: antifibrinolytic agents; fibrinolysis; hemorrhage; plasmin; α2-antiplasmin.
© 2019 International Society on Thrombosis and Haemostasis.
Figures
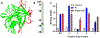

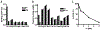
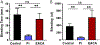
References
-
- Gall LS, Davenport RA. Fibrinolysis and antifibrinolytic treatment in the trauma patient. Current opinion in anaesthesiology. 2018; 31: 227–33. - PubMed
-
- Tsikouris JP, Suarez JA, Meyerrose GE. Plasminogen activator inhibitor-1: physiologic role, regulation, and the influence of common pharmacologic agents. J Clin Pharmacol. 2002; 42: 1187–99. - PubMed
-
- Lee KN, Lee CS, Tae WC, Jackson KW, Christiansen VJ, McKee PA. Cross-linking of wild-type and mutant alpha 2-antiplasmins to fibrin by activated factor XIII and by a tissue transglutaminase. J Biol Chem. 2000; 275: 37382–9. - PubMed
-
- Bajzar L Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler Thromb Vasc Biol. 2000; 20: 2511–8. - PubMed
-
- Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI-1 and alpha-antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007; 5: 812–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

