Genetic analyses led to the discovery of a super-active mutant of the RNA polymerase I
- PMID: 31136569
- PMCID: PMC6555540
- DOI: 10.1371/journal.pgen.1008157
Genetic analyses led to the discovery of a super-active mutant of the RNA polymerase I
Abstract
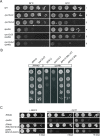
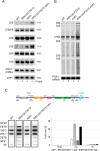
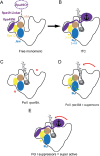
Most transcriptional activity of exponentially growing cells is carried out by the RNA Polymerase I (Pol I), which produces a ribosomal RNA (rRNA) precursor. In budding yeast, Pol I is a multimeric enzyme with 14 subunits. Among them, Rpa49 forms with Rpa34 a Pol I-specific heterodimer (homologous to PAF53/CAST heterodimer in human Pol I), which might be responsible for the specific functions of the Pol I. Previous studies provided insight in the involvement of Rpa49 in initiation, elongation, docking and releasing of Rrn3, an essential Pol I transcription factor. Here, we took advantage of the spontaneous occurrence of extragenic suppressors of the growth defect of the rpa49 null mutant to better understand the activity of Pol I. Combining genetic approaches, biochemical analysis of rRNA synthesis and investigation of the transcription rate at the individual gene scale, we characterized mutated residues of the Pol I as novel extragenic suppressors of the growth defect caused by the absence of Rpa49. When mapped on the Pol I structure, most of these mutations cluster within the jaw-lobe module, at an interface formed by the lobe in Rpa135 and the jaw made up of regions of Rpa190 and Rpa12. In vivo, the suppressor allele RPA135-F301S restores normal rRNA synthesis and increases Pol I density on rDNA genes when Rpa49 is absent. Growth of the Rpa135-F301S mutant is impaired when combined with exosome mutation rrp6Δ and it massively accumulates pre-rRNA. Moreover, Pol I bearing Rpa135-F301S is a hyper-active RNA polymerase in an in vitro tailed-template assay. We conclude that RNA polymerase I can be engineered to produce more rRNA in vivo and in vitro. We propose that the mutated area undergoes a conformational change that supports the DNA insertion into the cleft of the enzyme resulting in a super-active form of Pol I.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

