Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus
- PMID: 31136634
- PMCID: PMC6538184
- DOI: 10.1371/journal.pone.0217504
Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and Methicillin-resistant Staphylococcus aureus
Abstract
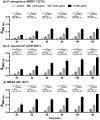
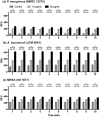
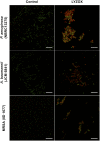
The recent emergence of antibiotic-resistant bacteria requires the development of new antibiotics or new agents capable of enhancing antibiotic activity. This study evaluated the antibacterial activity of lysozyme-chitosan oligosaccharide conjugates (LYZOX) against Pseudomonas aeruginosa, Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus (MRSA), which should resolve the problem of antibiotic-resistant bacteria. Bactericidal tests showed that LYZOX killed 50% more P. aeruginosa (NBRC 13275), A. baumannii and MRSA than the control treatment after 60 min. In addition, LYZOX was shown to inhibit the growth of P. aeruginosa (NBRC 13275 and PAO1), A. baumannii and MRSA better than its components. To elucidate the antibacterial mechanism of LYZOX, we performed cell membrane integrity assays, N-phenyl-1-naphthylamine assays, 2-nitrophenyl β-D-galactopyranoside assays and confocal laser scanning microscopy. These results showed that LYZOX affected bacterial cell walls and increased the permeability of the outer membrane and the plasma membrane. Furthermore, each type of bacteria treated with LYZOX was observed by electron microscopy. Electron micrographs revealed that these bacteria had the morphological features of both lysozyme-treated and chitosan oligosaccharide-treated bacteria and that LYZOX destroyed bacterial cell walls, which caused the release of intracellular contents from cells. An acquired drug resistance test revealed that these bacteria were not able to acquire resistance to LYZOX. The hemolytic toxicity test demonstrated the low hemolytic activity of LYZOX. In conclusion, LYZOX exhibited antibacterial activity and low drug resistance in the presence of P. aeruginosa, A. baumannii and MRSA and showed low hemolytic toxicity. LYZOX affected bacterial membranes, leading to membrane disruption and the release of intracellular contents and consequent bacterial cell death. LYZOX may serve as a novel candidate drug that could be used for the control of refractory infections.
Conflict of interest statement
A. Saito. and H. K. are employees of Wako Filter Technology Co., Ltd. Y. M. and D. M. are funded by Wako Filter Technology Co., Ltd. The rest of the authors have no conflicts of interest to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures








Similar articles
-
Antibacterial activity of lysozyme-chitosan oligosaccharide conjugates on two periodontal bacteria.Oral Dis. 2024 May;30(4):2728-2735. doi: 10.1111/odi.14710. Epub 2023 Aug 21. Oral Dis. 2024. PMID: 37602931
-
Trimethyl chitosan-capped silver nanoparticles with positive surface charge: Their catalytic activity and antibacterial spectrum including multidrug-resistant strains of Acinetobacter baumannii.Colloids Surf B Biointerfaces. 2017 Jul 1;155:61-70. doi: 10.1016/j.colsurfb.2017.03.054. Epub 2017 Apr 1. Colloids Surf B Biointerfaces. 2017. PMID: 28411476
-
Antibacterial mechanism of Pseudomonas aeruginosa UKMP14T rhamnolipids against multidrug resistant Acinetobacter baumannii.Microb Pathog. 2024 Aug;193:106743. doi: 10.1016/j.micpath.2024.106743. Epub 2024 Jun 13. Microb Pathog. 2024. PMID: 38879138
-
Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting.Curr Opin Infect Dis. 2005 Aug;18(4):306-13. doi: 10.1097/01.qco.0000171920.44809.f0. Curr Opin Infect Dis. 2005. PMID: 15985826 Review.
-
Minocycline--an old drug for a new century: emphasis on methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii.Int J Antimicrob Agents. 2009 Nov;34(5):395-401. doi: 10.1016/j.ijantimicag.2009.06.021. Epub 2009 Aug 8. Int J Antimicrob Agents. 2009. PMID: 19665876 Review.
Cited by
-
An Extensive Review of Natural Polymers Used as Coatings for Postharvest Shelf-Life Extension: Trends and Challenges.Polymers (Basel). 2021 Sep 25;13(19):3271. doi: 10.3390/polym13193271. Polymers (Basel). 2021. PMID: 34641086 Free PMC article. Review.
-
Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles.Antibiotics (Basel). 2021 Oct 19;10(10):1269. doi: 10.3390/antibiotics10101269. Antibiotics (Basel). 2021. PMID: 34680849 Free PMC article.
-
Anti-COVID-19 Credentials of Chitosan Composites and Derivatives: Future Scope?Antibiotics (Basel). 2023 Mar 28;12(4):665. doi: 10.3390/antibiotics12040665. Antibiotics (Basel). 2023. PMID: 37107027 Free PMC article. Review.
-
Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance.Antibiotics (Basel). 2022 Mar 15;11(3):390. doi: 10.3390/antibiotics11030390. Antibiotics (Basel). 2022. PMID: 35326853 Free PMC article. Review.
-
Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode.Microorganisms. 2020 Mar 13;8(3):401. doi: 10.3390/microorganisms8030401. Microorganisms. 2020. PMID: 32182971 Free PMC article.
References
-
- J ON. Tackling drug-resistant infections globally: final report and recommendations 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c....
-
- Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13(1):89–95. Epub 2001/01/13. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

