Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis
- PMID: 31136680
- PMCID: PMC6538309
- DOI: 10.1002/14651858.CD001176.pub4
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis
Update in
-
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis.Cochrane Database Syst Rev. 2019 Nov 30;11(11):CD001176. doi: 10.1002/14651858.CD001176.pub5. Cochrane Database Syst Rev. 2019. PMID: 31785173 Free PMC article.
Abstract
Background: Pouchitis occurs in approximately 50% of patients following ileal pouch-anal anastomosis (IPAA) for chronic ulcerative colitis (UC).
Objectives: The primary objective was to determine the efficacy and safety of medical therapies for prevention or treatment of acute or chronic pouchitis.
Search methods: We searched MEDLINE, Embase and CENTRAL from inception to 25 July 2018. We also searched references, trials registers, and conference proceedings.
Selection criteria: Randomized controlled trials of prevention or treatment of acute or chronic pouchitis in adults who underwent IPAA for UC were considered for inclusion.
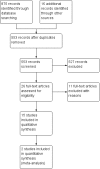
Data collection and analysis: Two authors independently screened studies for eligibility, extracted data and assessed the risk of bias. The certainty of the evidence was evaluated using GRADE. The primary outcome was clinical improvement or remission in participants with acute or chronic pouchitis, or the proportion of participants with no episodes of pouchitis after IPAA. Adverse events (AEs) was a secondary outcome. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for each dichotomous outcome.
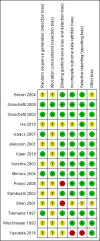
Main results: Fifteen studies (547 participants) were included. Four studies assessed treatment of acute pouchitis. Five studies assessed treatment of chronic pouchitis. Six studies assessed prevention of pouchitis. Three studies were low risk of bias. Three studies were high risk of bias and the other studies were unclear.Acute pouchitis: All ciprofloxacin participants (7/7) achieved remission at two weeks compared to 33% (3/9) of metronidazole participants (RR 2.68, 95% CI 1.13 to 6.35, very low certainty evidence). No ciprofloxacin participants (0/7) had an AE compared to 33% (3/9) of metronidazole participants (RR 0.18, 95% CI 0.01 to 2.98; very low certainty evidence). AEs included vomiting, dysgeusia or transient peripheral neuropathy. Forty-three per cent (6/14) of metronidazole participants achieved remission at 6 weeks compared to 50% (6/12) of budesonide enema participants (RR 0.86, 95% CI 0.37 to 1.96, very low certainty evidence). Fifty per cent (7/14) of metronidazole participants improved clinically at 6 weeks compared to 58% (7/12) of budesonide enema participants (RR 0.86, 95% CI 0.42 to 1.74, very low certainty evidence). Fifty-seven per cent (8/14) of metronidazole participants had an AE compared to 25% (3/12) of budesonide enema participants (RR 2.29, 95% CI 0.78 to 6.73, very low certainty evidence). AEs included anorexia, nausea, headache, asthenia, metallic taste, vomiting, paraesthesia, and depression. Twenty-five per cent (2/8) of rifaximin participants achieved remission at 4 weeks compared to 0% (0/10) of placebo participants (RR 6.11, 95% CI 0.33 to 111.71, very low certainty evidence). Thirty-eight per cent (3/8) of rifaximin participants improved clinically at 4 weeks compared to 30% (3/10) of placebo participants (RR 1.25, 95% CI 0.34 to 4.60, very low certainty evidence). Seventy-five per cent (6/8) of rifaximin participants had an AE compared to 50% (5/10) of placebo participants (RR 1.50, 95% CI 0.72 to 3.14, very low certainty evidence). AEs included diarrhea, flatulence, nausea, proctalgia, vomiting, thirst, candida, upper respiratory tract infection, increased hepatic enzyme, and cluster headache. Ten per cent (1/10) of Lactobacillus GG participants improved clinically at 12 weeks compared to 0% (0/10) of placebo participants (RR 3.00, 95% CI 0.14 to 65.90, very low certainty evidence).Chronic pouchitis: Eighty-five per cent (34/40) of De Simone Formulation participants maintained remission at 9 to 12 months compared to 3% (1/36) of placebo participants (RR 20.24, 95% CI 4.28 to 95.81, 2 studies; low certainty evidence). Two per cent (1/40) of De Simone Formulation participants had an AE compared to 0% (0/36) of placebo participants (RR 2.43, 95% CI 0.11 to 55.89; low certainty evidence). AEs included abdominal cramps, vomiting and diarrhea. Fifty per cent (3/6) of adalimumab patients achieved clinical improvement at 4 weeks compared to 43% (3/7) of placebo participants (RR, 1.17, 95% CI 0.36 to 3.76, low certainty evidence). Sixty per cent (6/10) of glutamine participants maintained remission at 3 weeks compared to 33% (3/9) of butyrate participants (RR 1.80, 95% CI 0.63 to 5.16, very low certainty evidence). Forty-five per cent (9/20) of patients treated with bismuth carbomer foam enema improved clinically at 3 weeks compared to 45% (9/20) of placebo participants (RR 1.00, 95% CI 0.50 to 1.98, very low certainty evidence). Twenty-five per cent (5/20) of participants in the bismuth carbomer foam enema group had an AE compared to 35% (7/20) of placebo participants (RR 0.71, 95% CI 0.27 to 1.88, very low certainty evidence). Adverse events included diarrhea, worsening symptoms, cramping, sinusitis, and abdominal pain.
Prevention: At 12 months, 90% (18/20) of De Simone Formulation participants had no episodes of acute pouchitis compared to 60% (12/20) of placebo participants (RR 1.50, 95% CI 1.02 to 2.21, low certainty evidence). Another study found 100% (16/16) of De Simone Formulation participants had no episodes of acute pouchitis at 12 months compared to 92% (11/12) of the no treatment control group (RR 1.10, 95% 0.89 to 1.36, very low certainty evidence). Eighty-six per cent (6/7) of Bifidobacterium longum participants had no episodes of acute pouchitis at 6 months compared to 60% (3/5) of placebo participants (RR 1.43, 95% CI 0.66 to 3.11, very low certainty evidence). Eleven per cent (1/9) of Clostridium butyricum MIYAIRI participants had no episodes of acute pouchitis at 24 months compared to 50% (4/8) of placebo participants (RR 0.22, 95% CI 0.03 to 1.60, very low certainty evidence). Forty-six per cent (43/94) of allopurinol participants had no episodes of pouchitis at 24 months compared to 43% (39/90) of placebo participants (RR 1.06, 95% CI 0.76 to 1.46; low certainty evidence). Eighty-one per cent (21/26) of tinidazole participants had no episodes of pouchitis over 12 months compared to 58% (7/12) of placebo participants (RR 1.38, 95% CI 0.83 to 2.31, very low certainty evidence).
Authors' conclusions: The effects of antibiotics, probiotics and other interventions for treating and preventing pouchitis are uncertain. Well designed, adequately powered studies are needed to determine the optimal therapy for the treatment and prevention of pouchitis.
Conflict of interest statement
Nghia Nguyen: None known
Bing Zhang: None known
Stefan D Holubar: None known
Darrell S Pardi: has received consulting fees from Assembly Bioscience, C3 Jian, Ferring, Janssen, Nestle, Merck, Otsuka, Salix, Seres, Gilead; research grants from Atlantic, Finch, Janssen, Merck, Pfizer, Salix, Seres, Takeda, Vedanta
Siddharth Singh ‐ has received consulting fees from AbbVie, Takeda, AMAG Pharmaceuticals; research grants from Pfizer and AbbVie; and honorarium for grant review from Pfizer.
Figures







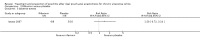







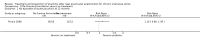



Update of
-
Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis.Cochrane Database Syst Rev. 2015 Nov 23;(11):CD001176. doi: 10.1002/14651858.CD001176.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 May 28;5:CD001176. doi: 10.1002/14651858.CD001176.pub4. PMID: 26593456 Free PMC article. Updated.
References
References to studies included in this review
-
- Brown SJ, Megan J, Smith S, Matchet D, Elliott R. Bifidobacterium longum BB‐536 and prevention of acute pouchitis. Gastroenterology 2004;126(4 Suppl 2):S465.
-
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119(2):305‐9. - PubMed
-
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double‐blind, placebo‐controlled trial. Gastroenterology 2003;124(5):1202‐9. - PubMed
-
- Ha CY, Bauer JJ, Lazarev M, Swaminath A, Sparrow M, Murphy SJ, et al. Early institution of tinidazole may prevent pouchitis following ileal pouch‐anal anastomosis (IPAA) surgery in ulcerative colitis (UC) patients. Gastroenterology 2010;138(5 Suppl 1):S69.
-
- Isaacs KL, Sandler RS, Abreu M, Picco MF, Hanauer SB, Bickston SJ, et al. Rifaximin for the treatment of active pouchitis: a randomized, double‐blind, placebo‐controlled pilot study. Inflammatory Bowel Diseases 2007;13(10):1250‐5. - PubMed
References to studies excluded from this review
-
- Bengtsson J, Adlerberth I, Östblom A, Saksena P, Öresland T, Börjesson L. Effect of probiotics (Lactobacillus plantarum 299 plus Bifidobacterium Cure21) in patients with poor ileal pouch function: a randomised controlled trial.. Scandinavian Journal of Gastroenterology 2016;51(9):1087‐92. [PUBMED: 27150635 ] - PubMed
-
- Laake KO, Bjørneklett A, Aamodt G, Aabakken L, Jacobsen M, Bakka A, et al. Outcome of four weeks' intervention with probiotics on symptoms and endoscopic appearance after surgical reconstruction with a J‐configurated ileal‐pouch‐anal‐anastomosis in ulcerative colitis. Scandinavian Journal of Gastroenterology 2005;40(1):43‐51. - PubMed
-
- Madden MV, McIntyre AS, Nicholls RJ. Double‐blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Digestive Diseases and Sciences 1994;39(6):1193‐6. - PubMed
-
- McLeod RS, Taylor DW, Cohen Z, Cullen JB. Single‐patient randomised clinical trial. Use in determining optimum treatment for patients with inflammation of Kock continent ileostomy reservoir. Lancet 1986;1(8483):726‐8. - PubMed
Additional references
-
- Boerr LA, Sambuelli AM, Sugai E, Graziano A, Valero J, Kogan Z, et al. Faecal alpha‐1‐antitrypsin concentration in the diagnosis and management of patients with pouchitis. European Journal of Gastroenterology and Hepatology 1995;7(2):129‐33. - PubMed
-
- Boerr LA, Sambuelli AM, Filinger E, Peredo A, Graziano A, Valero J, et al. Increased mucosal levels of leukotriene B4 in pouchitis: evidence for a persistent inflammatory state. European Journal of Gastroenterology and Hepatology 1996;8(1):57‐61. - PubMed
-
- Bonello JC, Thow GB, Manson RR. Mucosal enteritis: a complication of the continent ileostomy. Diseases of the Colon and Rectum 1981;24(1):37‐41. - PubMed
-
- Bär F, Kühbacher T, Dietrich NA, Krause T, Stallmach A, Teich N, et al. Vedolizumab in the treatment of chronic, antibiotic‐dependent or refractory pouchitis.. Alimentary Pharmacology and Therapeutics 2018;47(5):581‐7. [PUBMED: 29266360] - PubMed
-
- Chopra A, Pardi DS, Loftus EV Jr, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice.. Inflammatory Bowel Diseases 2006;12(1):29‐32. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

