Imiquimod and interferon-alpha augment monocyte-mediated astrocyte secretion of MCP-1, IL-6 and IP-10 in a human co-culture system
- PMID: 31136945
- PMCID: PMC6684287
- DOI: 10.1016/j.jneuroim.2019.576969
Imiquimod and interferon-alpha augment monocyte-mediated astrocyte secretion of MCP-1, IL-6 and IP-10 in a human co-culture system
Abstract
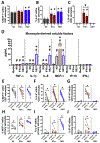
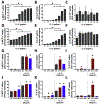
Toll-like receptor 7 (TLR7)-activation has been implicated as a significant mechanism of neuroinflammation triggered by ssRNA viruses. Infiltration of monocytes into the brain and astrocyte activation occurs during in vivo TLR7-mediated neuroinflammation. The objective here was to determine whether the TLR7 agonist, imiquimod, and interferon-alpha (IFN-α), promote monocyte-mediated astrocyte secretion of pro-inflammatory factors. Using a human primary co-culture system, we demonstrate that monocytes, together with imiquimod and IFN-α, promote astrocyte secretion of MCP-1, IL-6 and IP-10. Furthermore, TLR7-induced monocyte-derived IL-1β is critical for promoting the astrocyte response. Overall, this study provides a potential mechanism for TLR7-mediated neuroinflammation.
Keywords: Astrocyte; Co-culture; IL-1beta; Imiquimod; Interferon-alpha; Monocyte.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



References
-
- Abbott NJ, Ronnback L & Hansson E 2006. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci, 7, 41–53. - PubMed
-
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G & Peschle C 1992. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol, 149, 2358–66. - PubMed
-
- Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS, Singer EJ, Campbell TB, Mcmahon DD, Buchthal S, Cohen R, Yiannoutsos C, Letendre SL, Navia BA & Consortium HIVN 2015. Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr, 69, 29–35. - PMC - PubMed
-
- Andjelkovic AV, Kerkovich D & Pachter JS 2000. Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types. JLeukoc Biol, 68, 545–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

