Facile and Controllable Fabrication of Protein-Only Nanoparticles through Photo-Induced Crosslinking of Albumin and Their Application as DOX Carriers
- PMID: 31137647
- PMCID: PMC6566423
- DOI: 10.3390/nano9050797
Facile and Controllable Fabrication of Protein-Only Nanoparticles through Photo-Induced Crosslinking of Albumin and Their Application as DOX Carriers
Abstract
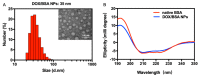
Protein-based nanoparticles, as an alternative to conventional polymer-based nanoparticles, offer great advantages in biomedical applications owing to their functional and biocompatible characteristics. However, the route of fabrication towards protein-based nanoparticles faces substantial challenges, including limitations in size control and unavoidable usage of toxic crosslinkers or organic solvents, which may raise safety concerns related to products and their degradation components. In the present study, a photo-induced crosslinking approach was developed to prepare stable, size-controlled protein-only nanoparticles. The facile one-step reaction irradiated by visible light enables the formation of monodispersed bovine serum albumin nanoparticles (BSA NPs) within several minutes through a tyrosine photo-redox reaction, requiring no cross-linking agents. The size of the BSA NPs could be precisely manipulated (from 20 to 100 nm) by controlling the duration time of illumination. The resultant BSA NPs exhibited spherical morphology, and the α-helix structure in BSA was preserved. Further study demonstrated that the 35 nm doxorubicin (DOX)-loaded BSA NPs achieved a drug loading content of 6.3%, encapsulation efficiency of 70.7%, and a controlled release profile with responsivity to both pH and reducing conditions. Importantly, the in vitro drug delivery experiment demonstrated efficient cellular internalizations of the DOX-loaded BSA NPs and inhibitory activities on MCF-7 and HeLa cells. This method shows the promise of being a platform for the green synthesis of protein-only nanoparticles for biomedical applications.
Keywords: BSA; biomedical application; drug delivery; photo-induced crosslinking; protein-only nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

