NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry
- PMID: 31137659
- PMCID: PMC6571951
- DOI: 10.3390/molecules24101995
NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry
Abstract
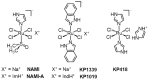
NAMI-A ((ImH)[trans-RuCl4(dmso-S)(Im)], Im = imidazole) and KP1019/1339 (KP1019 = (IndH)[trans-RuCl4(Ind)2], Ind = indazole; KP1339 = Na[trans-RuCl4(Ind)2]) are two structurally related ruthenium(III) coordination compounds that have attracted a lot of attention in the medicinal inorganic chemistry scientific community as promising anticancer drug candidates. This has led to a considerable amount of studies on their respective chemico-biological features and to the eventual admission of both to clinical trials. The encouraging pharmacological performances qualified KP1019 mainly as a cytotoxic agent for the treatment of platinum-resistant colorectal cancers, whereas the non-cytotoxic NAMI-A has gained the reputation of being a very effective antimetastatic drug. A critical and strictly comparative analysis of the studies conducted so far on NAMI-A and KP1019 allows us to define the state of the art of these experimental ruthenium drugs in terms of the respective pharmacological profiles and potential clinical applications, and to gain some insight into the inherent molecular mechanisms. Despite their evident structural relatedness, deeply distinct biological and pharmacological profiles do emerge. Overall, these two iconic ruthenium complexes form an exemplary and unique case in the field of medicinal inorganic chemistry.
Keywords: activation; anticancer; antimetastasis; aquation; biodistribution; clinical study; protein binding; ruthenium; uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Keppler B.K., Lipponer K.-G., Stenzel B., Kratz F. In: New tumor-inhibiting ruthenium complexes In Metal Complexes in Cancer Chemotherapy. Keppler B.K., editor. VCH; Weinheim, Germany: 1993. pp. 187–220.
-
- Hartinger C.G., Jakupec M.A., Zorbas-Seifried S., Groessl M., Egger A., Berger W., Zorbas H., Dyson P.J., Keppler B.K. KP1019, A New Redox-Active Anticancer Agent – Preclinical Development and Results of a Clinical Phase I Study in Tumor Patients. Chem. Biodiversity. 2008;5:2140–2155. doi: 10.1002/cbdv.200890195. - DOI - PubMed
-
- Trondl R., Heffeter P., Kowol C.R., Jakupec M.A., Berger W., Keppler B.K. NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application. Chem. Sci. 2014;5:2925–2932. doi: 10.1039/C3SC53243G. - DOI
-
- Mestroni G., Alessio E., Sava G., Pacor S., Coluccia M. The development of tumor-inhibiting ruthenium dimethylsulfoxide complexes. In: Keppler B.K., editor. Metal Complexes in Cancer Chemotherapy. VCH; Weinheim, Germany: 1993. pp. 157–185.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

