Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development
- PMID: 31137669
- PMCID: PMC6566554
- DOI: 10.3390/ijms20102555
Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development
Abstract
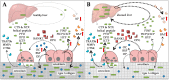
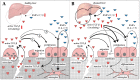
Almost all patients with chronic liver diseases (CLD) show altered bone metabolism. Depending on the etiology, this manifests in a severe osteoporosis in up to 75% of the affected patients. Due to high prevalence, the generic term hepatic osteodystrophy (HOD) evolved, describing altered bone metabolism, decreased bone mineral density, and deterioration of bone structure in patients with CLD. Once developed, HOD is difficult to treat and increases the risk of fragility fractures. Existing fractures affect the quality of life and, more importantly, long-term prognosis of these patients, which presents with increased mortality. Thus, special care is required to support the healing process. However, for early diagnosis (reduce fracture risk) and development of adequate treatment strategies (support healing of existing fractures), it is essential to understand the underlying mechanisms that link disturbed liver function with this bone phenotype. In the present review, we summarize proposed molecular mechanisms favoring the development of HOD and compromising the healing of associated fractures, including alterations in vitamin D metabolism and action, disbalances in transforming growth factor beta (TGF-β) and bone morphogenetic protein (BMP) signaling with histone deacetylases (HDACs) as secondary regulators, as well as alterations in the receptor activator of nuclear factor kappa B ligand (RANKL)-osteoprotegerin (OPG) system mediated by sclerostin. Based on these mechanisms, we give an overview on the limitations of early diagnosis of HOD with established serum markers.
Keywords: bone metabolism; bone morphogenetic proteins (BMPs); hepatic osteodystrophy; histone deacetylases (HDACs); liver disease; osteopenia; osteoporosis; sclerostin; transforming growth factor beta (TGF-β); vitamin D metabolism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Comment in
-
Highlight report: liver to bone communication.Arch Toxicol. 2019 Aug;93(8):2425-2426. doi: 10.1007/s00204-019-02518-2. Epub 2019 Jul 9. Arch Toxicol. 2019. PMID: 31286147 No abstract available.
Similar articles
-
Hepatic osteodystrophy: does the osteoprotegerin/receptor activator of nuclear factor-kB ligand system play a role?J Endocrinol Invest. 2005 Sep;28(8):677-82. doi: 10.1007/BF03347549. J Endocrinol Invest. 2005. PMID: 16277162
-
Hepatic osteodystrophy.Trop Gastroenterol. 2010 Apr-Jun;31(2):82-6. Trop Gastroenterol. 2010. PMID: 20862980 Review.
-
Liver disease and osteoporosis.Nutr Clin Pract. 2006 Jun;21(3):273-8. doi: 10.1177/0115426506021003273. Nutr Clin Pract. 2006. PMID: 16772544 Review.
-
Hepatic Osteodystrophy: A Global (Re)View of the Problem.Acta Clin Croat. 2017 Sep;56(3):512-525. doi: 10.20471/acc.2017.56.03.19. Acta Clin Croat. 2017. PMID: 29479918 Review.
-
The RANKL/OPG system and bone mineral density in patients with chronic liver disease.J Hepatol. 2005 Dec;43(6):973-83. doi: 10.1016/j.jhep.2005.05.034. Epub 2005 Jul 5. J Hepatol. 2005. PMID: 16143421
Cited by
-
Endocrine Regulation of Extra-skeletal Organs by Bone-derived Secreted Protein and the effect of Mechanical Stimulation.Front Cell Dev Biol. 2021 Nov 24;9:778015. doi: 10.3389/fcell.2021.778015. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901023 Free PMC article. Review.
-
Common musculoskeletal disorders in chronic liver disease patients.Jt Dis Relat Surg. 2021;32(3):818-823. doi: 10.52312/jdrs.2021.25. Epub 2021 Nov 19. Jt Dis Relat Surg. 2021. PMID: 34842121 Free PMC article.
-
Direct‑acting antiviral treatment decreases serum undercarboxylated osteocalcin in male patients with chronic hepatitis C.Biomed Rep. 2022 Sep 1;17(5):84. doi: 10.3892/br.2022.1567. eCollection 2022 Nov. Biomed Rep. 2022. PMID: 36185786 Free PMC article.
-
Relationship between nonalcoholic fatty liver disease and bone mineral density in elderly Chinese.J Orthop Surg Res. 2023 Sep 13;18(1):679. doi: 10.1186/s13018-023-04168-8. J Orthop Surg Res. 2023. PMID: 37705028 Free PMC article.
-
Mendelian randomization to evaluate the causal relationship between liver enzymes and the risk of six specific bone and joint-related diseases.Front Immunol. 2023 Aug 16;14:1195553. doi: 10.3389/fimmu.2023.1195553. eCollection 2023. Front Immunol. 2023. PMID: 37662902 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

