Flow Hydrodediazoniation of Aromatic Heterocycles
- PMID: 31137676
- PMCID: PMC6572451
- DOI: 10.3390/molecules24101996
Flow Hydrodediazoniation of Aromatic Heterocycles
Abstract
Continuous flow processing was applied for the rapid replacement of an aromatic amino group with a hydride. The approach was applied to a range of aromatic heterocycles, confirming the wide scope and substituent-tolerance of the processes. Flow equipment was utilized and the process optimised to overcome the problematically-unstable intermediates that have restricted yields in previous studies relying on batch procedures. Various common organic solvents were investigated as potential hydride sources. The approach has allowed key structures, such as amino-pyrazoles and aminopyridines, to be deaminated in good yield using a purely organic-soluble system.
Keywords: continuous processing; deamination; flow chemistry; heterocylic; hydrodediazoniation; isopentyl nitrite.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


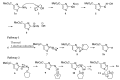
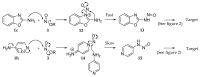
References
-
- Richter E., Loeffler J.E., Taylor E.C. Studies in Purine Chemistry. VIII. A Convenient Synthesis of Hypoxanthines and Adenines. J. Am. Chem. Soc. 1960;82:3144–3146. doi: 10.1021/ja01497a041. - DOI
-
- Meerwein H., Allendörfer H., Beekmann P., Kunert F., Morschel H., Pawellek F., Wunderlich K. Über die Reduktion aromatischer Diazoverbindungen mit äthern, 1.3-Dioxolanen und tertiären Aminen. Angew. Chemie. 1958;70:211–215. doi: 10.1002/ange.19580700804. - DOI
-
- Cadogan J.I.G., Molina G.A. A simple and convenient deamination of aromatic amines. J. Chem. Soc. Perkin Trans. 1. 1973:541. doi: 10.1039/p19730000541. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

