Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro
- PMID: 31138333
- PMCID: PMC6540573
- DOI: 10.1186/s40425-019-0622-0
Generation of monocyte-derived tumor-associated macrophages using tumor-conditioned media provides a novel method to study tumor-associated macrophages in vitro
Abstract
Background: Tumor-associated macrophages (TAM) are expanded and exhibit tumor-promoting properties within the tumor microenvironment. Current methods to study TAM have not been replicated across cancer types and often do not include exogenous growth factors from the tumor, a key factor in TAM differentiation in vivo.
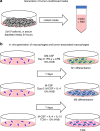
Methods: In this study, an in vitro method to generate monocyte- derived TAM using tumor- conditioned media (TCM) and a cytokine cocktail containing IL-4, IL-10, and M-CSF was utilized to study the phenotype, morphology, and function of TAM across multiple cancer types. TCM was generated from two breast cancer cell lines and an Epstein-Barr virus-positive lymphoma cell line. The properties of in vitro generated TAM were compared to in vitro generated M1 and M2- like macrophages and TAM isolated from patients with cancer.
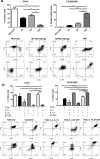
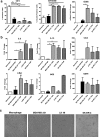
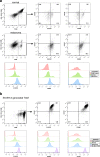
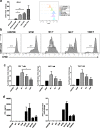
Results: TAM generated in this fashion displayed an increase in CD163/CD206 co-expression compared to M2- like macrophages (87 and 36%, respectively). TAM generated in vitro exhibited increased transcript levels of the functional markers IL-6, IL-10, CCL2, c-Myc, iNOS, and arginase compared to in vitro generated M2-like macrophages. Functionally, in vitro generated TAM inhibited the proliferation of T cells (47% decrease from M1-like macrophages) and the production of IFN-γ by natural killer cells was inhibited (44%) when co-cultured with TAM. Furthermore, in vitro generated TAM secreted soluble factors that promote the growth and survival of tumor cells.
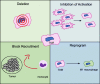
Conclusions: Limited access to patient TAM highlights the need for methods to generate TAM in vitro. Our data confirm that monocyte-derived TAM can be generated reliably using TCM plus the cytokine cocktail of IL-4, IL-10, and M-CSF. Given the ability of TAM to inhibit immune cell function, continued study of methods to deplete or deactivate TAM in the setting of cancer are warranted.
Keywords: Cancer; Immunotherapy; In vitro generation; Tumor-associated macrophages.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Fukuda K, Kobayashi A, Watabe K. The role of tumor-associated macrophage in tumor progression. Front Biosci (Schol Ed) 2012;4:787–798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
