Concentration-response studies of the chromosome-damaging effects of topoisomerase II inhibitors determined in vitro using human TK6 cells
- PMID: 31138411
- PMCID: PMC6570491
- DOI: 10.1016/j.mrgentox.2019.05.006
Concentration-response studies of the chromosome-damaging effects of topoisomerase II inhibitors determined in vitro using human TK6 cells
Abstract
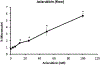
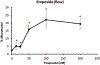
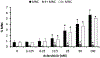
Topoisomerase II (topo II) inhibitors are commonly used as chemotherapy to treat multiple types of cancer, though their use is also associated with the development of therapy related acute leukemias. While the chromosome-damaging effects of etoposide, a topo II poison, have been proposed to act through a threshold mechanism, little is known about the chromosome damaging effects and dose responses for the catalytic inhibitors of the enzyme. The current study was designed to further investigate the potencies and concentration-response relationships of several topoisomerase II inhibitors, including the topoisomerase II poison etoposide, as well as catalytic inhibitors aclarubicin, merbarone, ICRF-154 and ICRF-187 using both a traditional in vitro micronucleus assay as well as a flow-cytometry based version of the assay. Benchmark dose (BMD) analysis was used to identify models that best fit the data and estimate a BMD, in this case the concentration at which a one standard deviation increase above the control frequency would be expected. All of the agents tested were potent in inducing micronuclei in human lymphoblastoid TK6 cells, with significant increases seen at low micromolar, and in the cases of aclarubicin and etoposide, at low nanomolar concentrations. Use of the anti-kinetochore CREST antibody with the microscopy-based assay demonstrated that the vast majority of the micronuclei originated from chromosome breakage. In comparing the two versions of the micronucleus assay, significant increases in micronucleated cells were observed at similar or lower concentrations using the traditional microscopy-based assay. BMD modeling of the data exhibited several advantages and proved to be a valuable alternative for concentration-response analysis, producing points of departure comparable to those derived using traditional no-observed or lowest-observed genotoxic effect level (NOGEL or LOGEL) approaches.
Keywords: Benchmark dose; Dose-response; Flow cytometry; Human cells; Micronucleus; Topoisomerase II inhibitors.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interest: None
Figures













Similar articles
-
Catalytic inhibitors of topoisomerase II are DNA-damaging agents: induction of chromosomal damage by merbarone and ICRF-187.Environ Mol Mutagen. 2002;39(4):348-56. doi: 10.1002/em.10072. Environ Mol Mutagen. 2002. PMID: 12112387
-
Cytogenetic evidence that DNA topoisomerase II is not involved in radiation induced chromsome-type aberrations.Mutat Res Genet Toxicol Environ Mutagen. 2015 Nov;793:14-8. doi: 10.1016/j.mrgentox.2015.06.006. Epub 2015 Jun 15. Mutat Res Genet Toxicol Environ Mutagen. 2015. PMID: 26520368
-
Efficient induction of chromosome-type aberrations by topoisomerase II inhibitors closely associated with stabilization of the cleavable complex in cultured fibroblastic cells.Mutat Res. 1995 May;328(2):151-61. doi: 10.1016/0027-5107(95)00005-4. Mutat Res. 1995. PMID: 7739599
-
Discovery of Novel Topoisomerase II Inhibitors by Medicinal Chemistry Approaches.J Med Chem. 2018 Oct 25;61(20):8947-8980. doi: 10.1021/acs.jmedchem.7b01202. Epub 2018 Jun 20. J Med Chem. 2018. PMID: 29870668 Review.
-
DNA topoisomerase II rescue by catalytic inhibitors: a new strategy to improve the antitumor selectivity of etoposide.Biochem Pharmacol. 1997 Oct 1;54(7):755-9. doi: 10.1016/s0006-2952(97)00116-0. Biochem Pharmacol. 1997. PMID: 9353129 Review.
References
-
- Mondrala S, Eastmond DA. Topoisomerase II inhibition by the bioactivated benzene metabolite hydroquinone involves multiple mechanisms. Chem Biol Interact. 2010;184: 259–268. - PubMed

