CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells
- PMID: 31138827
- PMCID: PMC6538758
- DOI: 10.1038/s41598-019-44333-w
CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells
Abstract
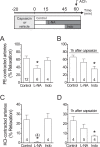
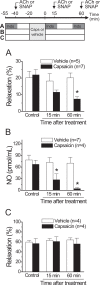
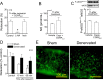
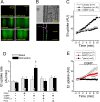
Blood flow distribution relies on precise coordinated control of vasomotor tone of resistance arteries by complex signalling interactions between perivascular nerves and endothelial cells. Sympathetic nerves are vasoconstrictors, whereas endothelium-dependent NO production provides a vasodilator component. In addition, resistance vessels are also innervated by sensory nerves, which are activated during inflammation and cause vasodilation by the release of calcitonin gene-related peptide (CGRP). Inflammation leads to superoxide anion (O2• -) formation and endothelial dysfunction, but the involvement of CGRP in this process has not been evaluated. Here we show a novel mechanistic relation between perivascular sensory nerve-derived CGRP and the development of endothelial dysfunction. CGRP receptor stimulation leads to pannexin-1-formed channel opening and the subsequent O2• --dependent connexin-based hemichannel activation in endothelial cells. The prolonged opening of these channels results in a progressive inhibition of NO production. These findings provide new therapeutic targets for the treatment of the inflammation-initiated endothelial dysfunction.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Lillo, M. A., Pérez, F. R., Puebla, M., Gaete, P. S. & Figueroa, X. F. In The Cardiovascular System - Physiology, Diagnostics and Clinical Implications (ed. David Gaze) (Intech, 2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

