Lectin microarray profiling and monosaccharide analysis of bovine milk immunoglobulin G oligosaccharides during the first 10 days of lactation
- PMID: 31139369
- PMCID: PMC6526632
- DOI: 10.1002/fsn3.950
Lectin microarray profiling and monosaccharide analysis of bovine milk immunoglobulin G oligosaccharides during the first 10 days of lactation
Abstract
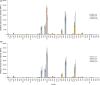
Immunoglobulin G (IgG) in bovine milk is credited with ensuring efficient passive immunity for newborn calves. Bovine milk IgG glycosylation may also have positive impacts on the health of nonbovine consumers of cow's milk. Milk IgG's glycosylation contributes to effector function and may also protect it from protease digestion, allowing IgG to reach the intestine for absorption. However, relatively little is known about changes in milk IgG oligosaccharide presentation and composition over early lactation. In this work, IgG was isolated from milk pooled from three cows at four time points over the first 10 days of lactation postparturition. Purified IgG was labeled with a fluorescent dye and interrogated with a microarray consisting of 48 carbohydrate-binding proteins (lectins) from plant, fungal, and bacterial sources. Lectin microarray profiles suggested that only subtle changes in the glycosylation of IgG occurred during days 2 and 3 of lactation, but by day 10, the lectin profile diverged from the other three time points. Monosaccharide analysis carried out after hydrolysis confirmed that the ratios of oligosaccharide components remained relatively stable through day 3 and also that sialylation was substantially reduced by day 10. The differences that were observed for glycosylation suggest that different functionalities associated with IgG glycosylation may be required in the first days of life.
Keywords: glycosylation; immunoglobulin; lactation; lectin; microarray; milk.
Conflict of interest statement
The authors of this work declare that they have no conflict of interest.
Figures


References
-
- Barboza, M. , Pinzon, J. , Wickramasinghe, S. , Froehlich, J. W. , Moeller, I. , Smilowitz, J. T. , … Medrano, J. F. (2012). Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria‐host interactions. Molecular and Cellular Proteomics, 11, M111–M015248. 10.1074/mcp.M111.015248 - DOI - PMC - PubMed
-
- Bondt, A. , Rombouts, Y. , Selman, M. H. , Hensbergen, P. J. , Reiding, K. R. , Hazes, J. M. , … Wuhrer, M. (2014). Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high‐throughput profiling method reveals pregnancy‐associated changes. Molecular and Cellular Proteomics, 13, 3029–3039. 10.1074/mcp.M114.039537 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

