Graphite-Conjugated Acids Reveal a Molecular Framework for Proton-Coupled Electron Transfer at Electrode Surfaces
- PMID: 31139719
- PMCID: PMC6535968
- DOI: 10.1021/acscentsci.9b00114
Graphite-Conjugated Acids Reveal a Molecular Framework for Proton-Coupled Electron Transfer at Electrode Surfaces
Abstract
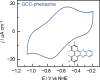
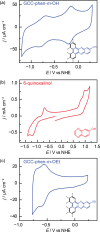
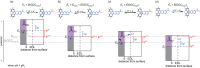
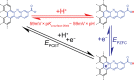
Proton-coupled electron-transfer (PCET) steps play a key role in energy conversion reactions. Molecular PCET reactions are well-described by "square schemes" in which the overall thermochemistry of the reaction is broken into its constituent proton-transfer and electron-transfer components. Although this description has been essential for understanding molecular PCET, no such framework exists for PCET reactions that take place at electrode surfaces. Herein, we develop a molecular square scheme framework for interfacial PCET by investigating the electrochemistry of molecularly well-defined acid/base sites conjugated to graphitic electrodes. Using cyclic voltammetry, we first demonstrate that, irrespective of the redox properties of the corresponding molecular analogue, proton transfer to graphite-conjugated acid/base sites is coupled to electron transfer. We then show that the thermochemistry of surface PCET events can be described by the pK a of the molecular analogue and the potential of zero free charge (zero-field reduction potential) of the electrode. This work provides a general framework for analyzing and predicting the thermochemistry of interfacial PCET reactions.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.N.J. and Y.S. are inventors on patent application 15/236,963, submitted by the Massachusetts Institute of Technology, that covers the graphite-conjugated materials described in this work.
Figures




References
-
- Roubelakis M. M.; Bediako D. K.; Dogutan D. K.; Nocera D. G. Proton-Coupled Electron Transfer Kinetics for the Hydrogen Evolution Reaction of Hangman Porphyrins. Energy Environ. Sci. 2012, 5 (7), 7737–7740. 10.1039/c2ee21123h. - DOI
-
- Rountree E. S.; Martin D. J.; McCarthy B. D.; Dempsey J. L. Linear Free Energy Relationships in the Hydrogen Evolution Reaction: Kinetic Analysis of a Cobaloxime Catalyst. ACS Catal. 2016, 6 (5), 3326–3335. 10.1021/acscatal.6b00667. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

