Chondroitin sulfate proteoglycan 4 enhanced melanoma motility and growth requires a cysteine in the core protein transmembrane domain
- PMID: 31140988
- PMCID: PMC6597303
- DOI: 10.1097/CMR.0000000000000574
Chondroitin sulfate proteoglycan 4 enhanced melanoma motility and growth requires a cysteine in the core protein transmembrane domain
Abstract
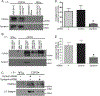
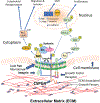
Chondroitin sulfate proteoglycan 4 (CSPG4) is a cell surface proteoglycan that enhances malignant potential in melanoma and several other tumor types. CSPG4 functions as a transmembrane scaffold in melanoma cells to activate oncogenic signaling pathways such as focal adhesion kinase (FAK) and extracellular signal regulated kinases 1,2, that control motility, invasion and anchorage independent growth. Here, we demonstrate that CSPG4 promotes directional motility and anchorage independent growth of melanoma cells by organizing and positioning a signaling complex containing activated FAK to lipid rafts within the plasma membrane of migrating cells. This FAK-containing signal transduction platform, which consists of syntenin-1, active Src and caveolin-1 requires the cytoplasmic domain of CSPG4 for assembly. Enhanced directional motility promoted by this complex also requires a CSPG4 transmembrane cysteine residue C2230. Substituting C2230 with alanine (CSPG4) still permits assembly of the signaling complex, however Src remains in an inactive state. CSPG4 also fails to promote anchorage independent growth and activation of extracellular signal regulated kinases 1,2. Therapies that target the transmembrane domain of CSPG4 could be a novel strategy for limiting progression by disrupting its function as a compartmentalized motogenic and growth-promoting oncogenic signaling node.
Conflict of interest statement
Figures




References
-
- Higgins SC, Bolteus AJ, Donovan LK, Hasegawa H, Doey L, Al Sarraj S, et al. Expression of the chondroitin sulphate proteoglycan, NG2, in paediatric brain tumors. Anticancer Res 2014; 34 (12):6919–6924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

