Mogamulizumab-Associated Cutaneous Granulomatous Drug Eruption Mimicking Mycosis Fungoides but Possibly Indicating Durable Clinical Response
- PMID: 31141114
- PMCID: PMC6547069
- DOI: 10.1001/jamadermatol.2019.0369
Mogamulizumab-Associated Cutaneous Granulomatous Drug Eruption Mimicking Mycosis Fungoides but Possibly Indicating Durable Clinical Response
Abstract
This case series report of mogamulizumab-associated cutaneous granulomatous drug eruption (CGDE) describes 12 patients with mycosis fungoides (MF) whose CGDE mimicked the MF but was followed by durable clinical MF response.
Conflict of interest statement
Figures
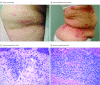
References
-
- Kim YH, Bagot M, Pinter-Brown L, et al. ; MAVORIC Investigators . Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. doi:10.1016/S1470-2045(18)30379-6 - DOI - PubMed
-
- Olsen EA, Whittaker S, Kim YH, et al. ; International Society for Cutaneous Lymphomas; United States Cutaneous Lymphoma Consortium; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer . Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. doi:10.1200/JCO.2010.32.0630 - DOI - PMC - PubMed

