Transformation of the National Breast Cancer Guideline Into Data-Driven Clinical Decision Trees
- PMID: 31141422
- PMCID: PMC7101250
- DOI: 10.1200/CCI.18.00150
Transformation of the National Breast Cancer Guideline Into Data-Driven Clinical Decision Trees
Abstract
Purpose: The essence of guideline recommendations often is intertwined in large texts. This impedes clinical implementation and evaluation and delays timely modular revisions needed to deal with an ever-growing amount of knowledge and application of personalized medicine. The aim of this project was to model guideline recommendations as data-driven clinical decision trees (CDTs) that are clinically interpretable and suitable for implementation in decision support systems.
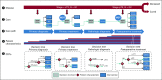
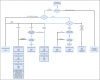
Methods: All recommendations of the Dutch national breast cancer guideline for nonmetastatic breast cancer were translated into CDTs. CDTs were constructed by nodes, branches, and leaves that represent data items (patient and tumor characteristics [eg, T stage]), data item values (eg, T2 or less), and recommendations (eg, chemotherapy), respectively. For all data items, source of origin was identified (eg, pathology), and where applicable, data item values were defined on the basis of existing classification and coding systems (eg, TNM, Breast Imaging Reporting and Data System, Systematized Nomenclature of Medicine). All unique routes through all CDTs were counted to measure the degree of data-based personalization of recommendations.
Results: In total, 60 CDTs were necessary to cover the whole guideline and were driven by 114 data items. Data items originated from pathology (49%), radiology (27%), clinical (12%), and multidisciplinary team (12%) reports. Of all data items, 101 (89%) could be classified by existing classification and coding systems. All 60 CDTs could be integrated in an interactive decision support app that contained 376 unique patient subpopulations.
Conclusion: By defining data items unambiguously and unequivocally and coding them to an international coding system, it was possible to present a complex guideline as systematically constructed modular data-driven CDTs that are clinically interpretable and accessible in a decision support app.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Mathijs P. Hendriks
Carolien H. Smorenburg
No other potential conflicts of interest were reported.
Figures


References
-
- Graham R, Mancher M, Miller Wolman D, et al (eds): Clinical Practice Guidelines We Can Trust. Washington, DC, National Academies Press, 2011. - PubMed
-
- Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: Do pathways work? Int J Qual Health Care. 2003;15:509–521. - PubMed
-
- Peleg M. Computer-interpretable clinical guidelines: A methodological review. J Biomed Inform. 2013;46:744–763. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

