Synthesis and Chemical Properties of 3-Phosphono-coumarins and 1,2-Benzoxaphosphorins as Precursors for Bioactive Compounds
- PMID: 31141889
- PMCID: PMC6600311
- DOI: 10.3390/molecules24112030
Synthesis and Chemical Properties of 3-Phosphono-coumarins and 1,2-Benzoxaphosphorins as Precursors for Bioactive Compounds
Abstract
Coumarins are an important class of natural heterocyclic compounds that have attracted considerable synthetic and pharmacological interest due to their various biological activities. This review emphasizes on the synthetic methods for the preparation of dialkyl 2-oxo-2H-1-benzo- pyran-3-phosphonates and alkyl 1,2-benzoxaphosphorin-3-carboxylates. Their chemical properties as acceptors in conjugate addition reactions, [2+2] and [3+2] cycloaddition reactions are discussed.
Keywords: 1,2-bezoxaphosphorines; 1,2-bezoxaphosphorines-3-phosphonic acid; 1,2-phosphorinines; 2-oxo-2H-1-benzopyrans; 2-oxo-2H-chromenes; [2+2] cycloaddition; [3+2] cycloaddition; conjugate addition; coumarins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
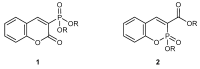






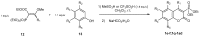
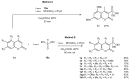
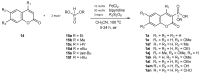


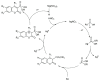



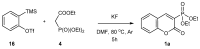




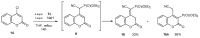


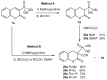







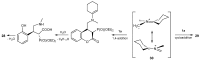
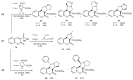











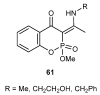
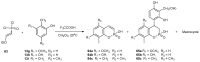




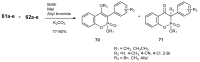






References
-
- Al-Majedy Y., Kadhum A.A., Ibraheem H., Al-Amiery A., Moneim A.A., Mohamad A.B. A Systematic Review on Pharmacological Activities of 4-Methylumbelliferon. Sys. Rev. Pharm. 2018;9:49–54. doi: 10.5530/srp.2018.1.10. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

