Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential
- PMID: 31141907
- PMCID: PMC6600424
- DOI: 10.3390/ijms20112611
Melatonin Reduces Excitability in Dorsal Root Ganglia Neurons with Inflection on the Repolarization Phase of the Action Potential
Abstract
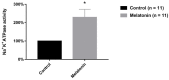
Melatonin is a neurohormone produced and secreted at night by pineal gland. Many effects of melatonin have already been described, for example: Activation of potassium channels in the suprachiasmatic nucleus and inhibition of excitability of a sub-population of neurons of the dorsal root ganglia (DRG). The DRG is described as a structure with several neuronal populations. One classification, based on the repolarizing phase of the action potential (AP), divides DRG neurons into two types: Without (N0) and with (Ninf) inflection on the repolarization phase of the action potential. We have previously demonstrated that melatonin inhibits excitability in N0 neurons, and in the present work, we aimed to investigate the melatonin effects on the other neurons (Ninf) of the DRG neuronal population. This investigation was done using sharp microelectrode technique in the current clamp mode. Melatonin (0.01-1000.0 nM) showed inhibitory activity on neuronal excitability, which can be observed by the blockade of the AP and by the increase in rheobase. However, we observed that, while some neurons were sensitive to melatonin effect on excitability (excitability melatonin sensitive-EMS), other neurons were not sensitive to melatonin effect on excitability (excitability melatonin not sensitive-EMNS). Concerning the passive electrophysiological properties of the neurons, melatonin caused a hyperpolarization of the resting membrane potential in both cell types. Regarding the input resistance (Rin), melatonin did not change this parameter in the EMS cells, but increased its values in the EMNS cells. Melatonin also altered several AP parameters in EMS cells, the most conspicuously changed was the (dV/dt)max of AP depolarization, which is in coherence with melatonin effects on excitability. Otherwise, in EMNS cells, melatonin (0.1-1000.0 nM) induced no alteration of (dV/dt)max of AP depolarization. Thus, taking these data together, and the data of previous publication on melatonin effect on N0 neurons shows that this substance has a greater pharmacological potency on Ninf neurons. We suggest that melatonin has important physiological function related to Ninf neurons and this is likely to bear a potential relevant therapeutic use, since Ninf neurons are related to nociception.
Keywords: DRG; action potential; dorsal root ganglion; excitability; melatonin; passive electric properties.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Melatonin decreases neuronal excitability in a sub-population of dorsal root ganglion neurons.Brain Res. 2018 Aug 1;1692:1-8. doi: 10.1016/j.brainres.2018.04.027. Epub 2018 Apr 24. Brain Res. 2018. PMID: 29702086
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Action Potential Broadening in Capsaicin-Sensitive DRG Neurons from Frequency-Dependent Reduction of Kv3 Current.J Neurosci. 2017 Oct 4;37(40):9705-9714. doi: 10.1523/JNEUROSCI.1703-17.2017. Epub 2017 Sep 6. J Neurosci. 2017. PMID: 28877968 Free PMC article.
-
Unexplained peculiarities of the dorsal root ganglion.Pain. 1999 Aug;Suppl 6:S27-S35. doi: 10.1016/S0304-3959(99)00135-9. Pain. 1999. PMID: 10491970 Review.
-
Diabetes-induced electrophysiological alterations on neurosomes in ganglia of peripheral nervous system.Biophys Rev. 2023 Jul 17;15(4):625-638. doi: 10.1007/s12551-023-01094-1. eCollection 2023 Aug. Biophys Rev. 2023. PMID: 37681090 Free PMC article. Review.
Cited by
-
Effects of Melatonin on Diabetic Neuropathy and Retinopathy.Int J Mol Sci. 2021 Dec 22;23(1):100. doi: 10.3390/ijms23010100. Int J Mol Sci. 2021. PMID: 35008523 Free PMC article. Review.
-
Melatonin from an Antioxidant to a Classic Hormone or a Tissue Factor: Experimental and Clinical Aspects 2019.Int J Mol Sci. 2020 May 21;21(10):3645. doi: 10.3390/ijms21103645. Int J Mol Sci. 2020. PMID: 32455655 Free PMC article.
-
Deficient AMPK activity contributes to hyperexcitability in peripheral nociceptive sensory neurons and thermal hyperalgesia in lupus mice.PLoS One. 2023 Jul 13;18(7):e0288356. doi: 10.1371/journal.pone.0288356. eCollection 2023. PLoS One. 2023. PMID: 37440542 Free PMC article.
-
Diabetes mellitus differently affects electrical membrane properties of vagal afferent neurons of rats.Physiol Rep. 2023 Feb;11(4):e15605. doi: 10.14814/phy2.15605. Physiol Rep. 2023. PMID: 36807809 Free PMC article.
-
Melatonin Successfully Rescues the Hippocampal Molecular Machinery and Enhances Anti-oxidative Activity Following Early-Life Sleep Deprivation Injury.Antioxidants (Basel). 2021 May 13;10(5):774. doi: 10.3390/antiox10050774. Antioxidants (Basel). 2021. PMID: 34068192 Free PMC article.
References
-
- Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587. doi: 10.1021/ja01543a060. - DOI
-
- Huang F., Guan X., Yan Y., Fan W., You Y., He H., Cheng B. Electrophysiological effects of melatonin on rat trigeminal ganglion neurons that participate in nociception in vitro. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3234–3239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

