Emergence of Fluoxetine-Resistant Variants during Treatment of Human Pancreatic Cell Cultures Persistently Infected with Coxsackievirus B4
- PMID: 31141921
- PMCID: PMC6630805
- DOI: 10.3390/v11060486
Emergence of Fluoxetine-Resistant Variants during Treatment of Human Pancreatic Cell Cultures Persistently Infected with Coxsackievirus B4
Abstract
This study reports the antiviral activity of the drug fluoxetine against some enteroviruses (EV). We had previously established a model of persistent coxsackievirus B4 (CVB4) infection in pancreatic cell cultures and demonstrated that fluoxetine could clear the virus from these cultures. We further report the emergence of resistant variants during the treatment with fluoxetine in this model. Four independent persistent CVB4 infections in Panc-1 cells were treated with fluoxetine. The resistance to fluoxetine was investigated in an acute infection model. The 2C region, the putative target of fluoxetine antiviral activity, was sequenced. However, Fluoxetine treatment failed to clear CVB4 in two persistent infections. The resistance to fluoxetine was later confirmed in HEp-2 cells. The decrease in viral titer was significantly lower when cells were inoculated with the virus obtained from persistently infected cultures treated with fluoxetine than those from susceptible mock-treated cultures (0.6 log TCID50/mL versus 4.2 log TCID50/mL, p < 0.0001). Some previously described mutations and additional ones within the 2C protein were found in the fluoxetine-resistant isolates. The model of persistent infection is an interesting tool for assessing the emergence of variants resistant to anti-EV molecules. The resistance of EV strains to fluoxetine and its mechanisms require further investigation.
Keywords: coxsackievirus B4; enteroviruses; fluoxetine; mutations; persistent infection; resistance.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
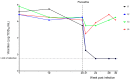
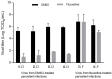

Similar articles
-
Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine.Antiviral Res. 2015 Apr;116:51-4. doi: 10.1016/j.antiviral.2015.01.010. Epub 2015 Feb 2. Antiviral Res. 2015. PMID: 25655448
-
Fluoxetine Inhibits Enterovirus Replication by Targeting the Viral 2C Protein in a Stereospecific Manner.ACS Infect Dis. 2019 Sep 13;5(9):1609-1623. doi: 10.1021/acsinfecdis.9b00179. Epub 2019 Jul 31. ACS Infect Dis. 2019. PMID: 31305993 Free PMC article.
-
Macrophages can transmit coxsackievirus B4 to pancreatic cells and can impair these cells.J Med Virol. 2024 Oct;96(10):e70009. doi: 10.1002/jmv.70009. J Med Virol. 2024. PMID: 39422382
-
Fluoxetine can inhibit coxsackievirus-B4 E2 in vitro and in vivo.Antiviral Res. 2018 Nov;159:130-133. doi: 10.1016/j.antiviral.2018.10.002. Epub 2018 Oct 2. Antiviral Res. 2018. PMID: 30290197
-
Modulation of proteolytic polyprotein processing by coxsackievirus mutants resistant to inhibitors targeting phosphatidylinositol-4-kinase IIIβ or oxysterol binding protein.Antiviral Res. 2017 Nov;147:86-90. doi: 10.1016/j.antiviral.2017.10.006. Epub 2017 Oct 9. Antiviral Res. 2017. PMID: 29024767
Cited by
-
Antiviral Agents: Discovery to Resistance.Viruses. 2020 Apr 7;12(4):406. doi: 10.3390/v12040406. Viruses. 2020. PMID: 32272550 Free PMC article.
-
Fluoxetine Can Inhibit SARS-CoV-2 In Vitro.Microorganisms. 2021 Feb 9;9(2):339. doi: 10.3390/microorganisms9020339. Microorganisms. 2021. PMID: 33572117 Free PMC article.
-
Fighting Enteroviral Infections to Prevent Type 1 Diabetes.Microorganisms. 2022 Apr 1;10(4):768. doi: 10.3390/microorganisms10040768. Microorganisms. 2022. PMID: 35456818 Free PMC article.
-
Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19.Front Pharmacol. 2021 Apr 20;12:652688. doi: 10.3389/fphar.2021.652688. eCollection 2021. Front Pharmacol. 2021. PMID: 33959018 Free PMC article.
References
-
- Knowles N., Hovi T., Hyypiä T. Picornaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego, CA, USA: 2012. pp. 855–880.
-
- Romero J.R. Pediatric group B coxsackievirus infections. Curr. Top. Microbiol. Immunol. 2008;323:223–239. - PubMed
-
- Chapman N.M., Kim K.S. Persistent coxsackievirus infection: Enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr. Top. Microbiol. Immunol. 2008;323:275–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

