Gastric Cancer Extracellular Vesicles Tune the Migration and Invasion of Epithelial and Mesenchymal Cells in a Histotype-Dependent Manner
- PMID: 31141946
- PMCID: PMC6600627
- DOI: 10.3390/ijms20112608
Gastric Cancer Extracellular Vesicles Tune the Migration and Invasion of Epithelial and Mesenchymal Cells in a Histotype-Dependent Manner
Abstract
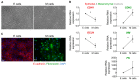
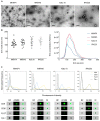
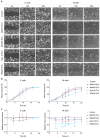
Extracellular vesicles (EVs) secreted by tumor cells modulate recipient cells' behavior, but their effects in normal cells from the tumor microenvironment remain poorly known. In this study, we dissected the functional impact of gastric cancer cell-derived EVs (GC-EVs), representative of distinct GC histotypes, on the behavior of normal isogenic epithelial and mesenchymal cells. GC-EVs were isolated by differential centrifugation and characterized by transmission electron microscopy, nanoparticle tracking analysis, and imaging flow-cytometry. Epithelial and mesenchymal cells were challenged with GC-EVs and submitted to proliferation, migration, and invasion assays. Expression of epithelial and mesenchymal markers was followed by immunofluorescence and flow-cytometry. Our results indicated that GC-EVs secreted by diffuse-type cancer cells decrease the migration of recipient cells. This effect was more prominent and persistent for mesenchymal recipient cells, which also increased Fibronectin expression in response to EVs. GC-EVs secreted by cancer cells derived from tumors with an intestinal component increased invasion of recipient epithelial cells, without changes in EMT markers. In summary, this study demonstrated that GC-EVs modulate the migration and invasion of epithelial and mesenchymal cells from the tumor microenvironment, in a histotype-dependent manner, highlighting new features of intestinal and diffuse-type GC cells, which may help explaining differential metastasis patterns and aggressiveness of GC histotypes.
Keywords: epithelial-to-mesenchymal transition; extracellular vesicles; gastric cancer; invasion; migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

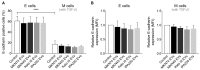
Similar articles
-
Mesenchymal stem cell‑derived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway.Int J Oncol. 2019 May;54(5):1843-1852. doi: 10.3892/ijo.2019.4747. Epub 2019 Mar 13. Int J Oncol. 2019. PMID: 30864702
-
Extracellular vesicles from retinal pigment epithelial cells expressing R345W-Fibulin-3 induce epithelial-mesenchymal transition in recipient cells.J Extracell Vesicles. 2023 Oct;12(10):e12373. doi: 10.1002/jev2.12373. J Extracell Vesicles. 2023. PMID: 37855063 Free PMC article.
-
Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients.Int J Mol Sci. 2019 Feb 22;20(4):953. doi: 10.3390/ijms20040953. Int J Mol Sci. 2019. PMID: 30813244 Free PMC article.
-
The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy.Mol Cancer. 2019 Mar 30;18(1):62. doi: 10.1186/s12943-019-0967-5. Mol Cancer. 2019. PMID: 30925929 Free PMC article. Review.
-
Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Epithelial-Mesenchymal Transition.Int J Mol Sci. 2019 Sep 27;20(19):4813. doi: 10.3390/ijms20194813. Int J Mol Sci. 2019. PMID: 31569731 Free PMC article. Review.
Cited by
-
The Ubiquitin Gene Expression Pattern and Sensitivity to UBB and UBC Knockdown Differentiate Primary 23132/87 and Metastatic MKN45 Gastric Cancer Cells.Int J Mol Sci. 2020 Jul 30;21(15):5435. doi: 10.3390/ijms21155435. Int J Mol Sci. 2020. PMID: 32751694 Free PMC article.
-
Integrated bioinformatics, network pharmacology, and artificial intelligence to predict the mechanism of celastrol against muscle atrophy caused by colorectal cancer.Front Genet. 2022 Nov 7;13:1012932. doi: 10.3389/fgene.2022.1012932. eCollection 2022. Front Genet. 2022. PMID: 36419834 Free PMC article.
-
Anti-Cancer Role and Therapeutic Potential of Extracellular Vesicles.Cancers (Basel). 2021 Dec 15;13(24):6303. doi: 10.3390/cancers13246303. Cancers (Basel). 2021. PMID: 34944923 Free PMC article. Review.
-
A Novel Model of Cancer Drug Resistance: Oncosomal Release of Cytotoxic and Antibody-Based Drugs.Biology (Basel). 2020 Mar 5;9(3):47. doi: 10.3390/biology9030047. Biology (Basel). 2020. PMID: 32150875 Free PMC article. Review.
References
-
- Zomer A., Maynard C., Verweij F.J., Kamermans A., Schafer R., Beerling E., Schiffelers R.M., de Wit E., Berenguer J., Ellenbroek S.I.J., et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- PTDC/BBB-ECT/2518/2014/Portuguese FCT
- NORTE-01-0145-FEDER-000029/Norte Portugal Regional Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF)
- POCI-01-0145-FEDER-007274/FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through Portuguese Science & Technology Foundation (FCT) /
- PD/BD/128406/2017 to SR; SFRH/BPD/86543/2012 to JC; SFRH/BPD/89764/2012 to PO/FCT fellowships
- BiotechHealth Programe (Doctoral Programme on Cellular and Molecular Biotechnology Applied to Health Sciences)/FCT PhD Programmes and by Programa Operacional Potencial Humano (POCH)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

