Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index than Sorafenib via Zebrafish Drug Screening Platform
- PMID: 31141996
- PMCID: PMC6628114
- DOI: 10.3390/cancers11060739
Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index than Sorafenib via Zebrafish Drug Screening Platform
Abstract
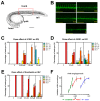
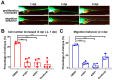
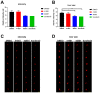
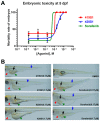
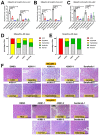
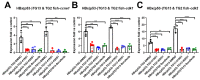
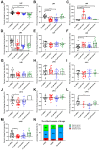
Hepatocellular carcinoma (HCC) ranks as the fourth leading cause of cancer-related deaths worldwide. Sorafenib was the only U.S. Food and Drug Administration (FDA) approved drug for treating advanced HCC until recently, so development of new target therapy is urgently needed. In this study, we established a zebrafish drug screening platform and compared the therapeutic effects of two multiple tyrosine kinase inhibitors, 419S1 and 420S1, with Sorafenib. All three compounds exhibited anti-angiogenesis abilities in immersed fli1:EGFP transgenic embryos and the half inhibition concentration (IC50) was determined. 419S1 exhibited lower hepatoxicity and embryonic toxicity than 420S1 and Sorafenib, and the half lethal concentration (LC50) was determined. The therapeutic index (LC50/IC50) for 419S1 was much higher than for Sorafenib and 420S1. The compounds were either injected retro-orbitally or by oral gavage to adult transgenic zebrafish with HCC. The compounds not only rescued the pathological feature, but also reversed the expression levels of cell-cycle-related genes and protein levels of a proliferation marker. Using a patient-derived-xenograft assay, we found that the effectiveness of 419S1 and 420S1 in preventing liver cancer proliferation is better than that of Sorafenib. With integrated efforts and the advantage of the zebrafish platform, we can find more effective and safe drugs for HCC treatment and screen for personalized medicine.
Keywords: anti-angiogenesis; anti-proliferation; hepatocellular carcinoma (HCC); patient-derived xenograft; zebrafish drug screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

