Illegitimate and Repeated Genomic Integration of Cell-Free Chromatin in the Aetiology of Somatic Mosaicism, Ageing, Chronic Diseases and Cancer
- PMID: 31142004
- PMCID: PMC6628102
- DOI: 10.3390/genes10060407
Illegitimate and Repeated Genomic Integration of Cell-Free Chromatin in the Aetiology of Somatic Mosaicism, Ageing, Chronic Diseases and Cancer
Abstract
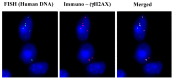
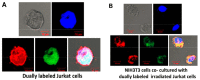
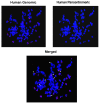
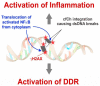
Emerging evidence suggests that an individual is a complex mosaic of genetically divergent cells. Post-zygotic genomes of the same individual can differ from one another in the form of single nucleotide variations, copy number variations, insertions, deletions, inversions, translocations, other structural and chromosomal variations and footprints of transposable elements. High-throughput sequencing has led to increasing detection of mosaicism in healthy individuals which is related to ageing, neuro-degenerative disorders, diabetes mellitus, cardiovascular diseases and cancer. These age-related disorders are also known to be associated with significant increase in DNA damage and inflammation. Herein, we discuss a newly described phenomenon wherein the genome is under constant assault by illegitimate integration of cell-free chromatin (cfCh) particles that are released from the billions of cells that die in the body every day. We propose that such repeated genomic integration of cfCh followed by dsDNA breaks and repair by non-homologous-end-joining as well as physical damage to chromosomes occurring throughout life may lead to somatic/chromosomal mosaicism which would increase with age. We also discuss the recent finding that genomic integration of cfCh and the accompanying DNA damage is associated with marked activation of inflammatory cytokines. Thus, the triple pathologies of somatic mosaicism, DNA/chromosomal damage and inflammation brought about by a common mechanism of genomic integration of cfCh may help to provide an unifying model for the understanding of aetiologies of the inter-related conditions of ageing, degenerative disorders and cancer.
Keywords: DNA damage; NFκB; apoptosis; cell death; chromosomal damage; chromosomal mosaicism; chromosomes; circulating nucleic acids; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
A New Perspective on the Origin of DNA Double-Strand Breaks and Its Implications for Ageing.Genes (Basel). 2021 Jan 26;12(2):163. doi: 10.3390/genes12020163. Genes (Basel). 2021. PMID: 33530310 Free PMC article. Review.
-
Prevention of radiation-induced bystander effects by agents that inactivate cell-free chromatin released from irradiated dying cells.Cell Death Dis. 2018 Nov 15;9(12):1142. doi: 10.1038/s41419-018-1181-x. Cell Death Dis. 2018. PMID: 30442925 Free PMC article.
-
Evidence for cell-free nucleic acids as continuously arising endogenous DNA mutagens.Mutat Res. 2016 Nov-Dec;793-794:15-21. doi: 10.1016/j.mrfmmm.2016.10.002. Epub 2016 Oct 12. Mutat Res. 2016. PMID: 27768916 Review.
-
Is inflammation a direct response to dsDNA breaks?Mutat Res. 2018 Mar;808:48-52. doi: 10.1016/j.mrfmmm.2018.02.002. Epub 2018 Feb 22. Mutat Res. 2018. PMID: 29518635
-
Cell-free chromatin: A newly described mediator of systemic inflammation.J Biosci. 2019 Jun;44(2):32. J Biosci. 2019. PMID: 31180045 Review.
Cited by
-
Origins and Consequences of Chromosomal Instability: From Cellular Adaptation to Genome Chaos-Mediated System Survival.Genes (Basel). 2020 Sep 30;11(10):1162. doi: 10.3390/genes11101162. Genes (Basel). 2020. PMID: 33008067 Free PMC article.
-
A novel pro-oxidant combination of resveratrol and copper reduces transplant related toxicities in patients receiving high dose melphalan for multiple myeloma (RESCU 001).PLoS One. 2022 Feb 4;17(2):e0262212. doi: 10.1371/journal.pone.0262212. eCollection 2022. PLoS One. 2022. PMID: 35120140 Free PMC article.
-
Cell-free chromatin particles released from dying host cells are global instigators of endotoxin sepsis in mice.PLoS One. 2020 Mar 4;15(3):e0229017. doi: 10.1371/journal.pone.0229017. eCollection 2020. PLoS One. 2020. PMID: 32130239 Free PMC article.
-
Copper Imparts a New Therapeutic Property to Resveratrol by Generating ROS to Deactivate Cell-Free Chromatin.Pharmaceuticals (Basel). 2025 Jan 20;18(1):132. doi: 10.3390/ph18010132. Pharmaceuticals (Basel). 2025. PMID: 39861193 Free PMC article.
-
Cytokine Storm as a Cellular Response to dsDNA Breaks: A New Proposal.Front Immunol. 2021 Feb 1;12:622738. doi: 10.3389/fimmu.2021.622738. eCollection 2021. Front Immunol. 2021. PMID: 33597956 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

