Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS
- PMID: 31142272
- PMCID: PMC6540456
- DOI: 10.1186/s12883-019-1330-6
Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS
Abstract
Background: Nutritional status is an important prognostic factor in Amyotrophic Lateral Sclerosis (ALS). We wished to study the safety, tolerability and efficacy of nutritional counseling with or without an mHealth application to maintain or increase body weight in ALS, compared to standard care.
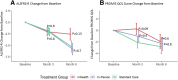
Methods: In this randomized open-label, standard-of-care-controlled, single-center clinical trial, we randomly assigned adults with ALS to one of three nutritional interventions: counseling by their physician or nurse ("standard care"), counseling by a registered dietitian (RD) ("in-person"), or counseling supported by a mHealth app ("mHealth"). Both intervention arms received tailored nutrition recommendations and recorded dietary intake and weight biweekly (mHealth) or monthly (in-person). The primary outcome of weight and secondary and tertiary outcomes of calorie intake, ALSFRS-R, and quality of life (QOL) were recorded at each clinic visit and analyzed in an ITT mixed model analysis.
Results: A total of 88 participants were enrolled of whom 78 were included in this analysis. The three arms were well-balanced except for excess males in the mHealth arm and greater weight lost at baseline in the in-person arm. Participants in the mHealth arm increased their calorie intake at month 3 to an average of 94% (95% CI: 85, 103) of recommended calories, compared to 81% (95% CI: 72, 91, p = 0.06 vs. mHealth) in the standard care arm. After 6 months, calorie intake was not different among the three arms. Overall weight was stable across all three groups. QOL scores in the mHealth arm were stable over 3 months (0.3 points, 95% CI: - 1.7, 2.2) compared to worsening in standard care (- 2.1 points, 95% CI: - 4.0, - 0.2, p = 0.09 vs. mHealth), but all scores declined by 6 months. ALSFRS-R total scores declined by an average of - 2.6 points (95% CI: - 5.1, - 0.1) over 6 months in the mHealth arm (p = 0.13 vs. standard care) compared to - 5.8 points (95% CI: - 8.2, - 3.4, p = 0.74 vs. standard care) in the in-person and - 5.2 points (95% CI: - 7.6, - 2.9) in the standard care arm.
Conclusions: Nutritional counseling by a registered dietitian (with or without support by an mHealth app) is safe but did not maintain weight significantly better than standard care in ALS patients.
Trial registration: https://clinicaltrials.gov/ identifier NCT02418546. Registered April 16, 2015.
Keywords: ALS; Amyotrophic lateral sclerosis; Mobile health technology; Neurodegenerative disease; Nutrition; Nutritional counseling; Randomized controlled trial; mHealth.
Conflict of interest statement
Anne-Marie Wills M.D., M.P.H. has received research funding from the ALS Association, has participated in clinical trials funded by Acorda, Biogen, Bristol-Myers Squibb, Sanofi/Genzyme, Pfizer and received consultant payments from Acorda, Mitsubishi Tanabe Pharma America, and Accordant, a CVS/Caremark disease management company.
Jamie Garry MS RD, reports grants from ALS Association, grants from NIH/NCRR, during the conduct of the study.
Jane Hubbard MS RD reports grants from ALS Association, grants from NIH/NCRR, during the conduct of the study.
Taylor Mezoian BS has nothing to disclose.
Christopher T. Breen BA has nothing to disclose.
Courtney Ortiz- Miller BA has nothing to disclose.
Paige Nalipinski MA SLP has nothing to disclose.
Stacey Sullivan MS SLP has nothing to disclose.
James Berry MD MPH, has been a consultant for MT Pharma, Denali Therapeutics, Anelixis Therapeutics, held a research fellowship position with Voyager Therapeutics, and been a Site Investigator for projects sponsored by Brainstorm Cell Therapeutics, Neuraltus, Cytokinetics, and Amylyx.
Merit Cudkowicz MD MSc. Has received grants from the NINDS, and received consulting funds from Lilly, MT Pharma, Orion, Cytokinetics, Biohaven, Waves and ImmunityPharm.
Sabrina Paganoni MD PhD, has received research funding from the ALS Association, the American Academy of Neurology, ALS Finding a Cure, the Salah Foundation, and Amylyx.
James Chan MA has nothing to disclose.
Eric A. Macklin PhD serves on Data and Safety Monitoring Boards for Acorda Therapeutics and Shire Human Genetic Therapies, received research funds and served on a Steering Committee for Acorda Therapeutics, and consulted for Myolex Inc. and Lavin Consulting.
Competing interests.
Figures


References
-
- Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, Preux PM, Couratier P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:628–634. doi: 10.1136/jnnp.2010.211474. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

