Novel sporozoite-based ELISpot assay to assess frequency of parasite-specific B cells after vaccination with irradiated sporozoites
- PMID: 31142328
- PMCID: PMC6540377
- DOI: 10.1186/s12936-019-2819-6
Novel sporozoite-based ELISpot assay to assess frequency of parasite-specific B cells after vaccination with irradiated sporozoites
Abstract
Background: Whole parasite vaccination is an efficacious strategy to induce sterile immunity and to prevent malaria transmission. Understanding the mechanism and response of immune cells to vaccines plays a critical role in deciphering correlates of protection against infection and disease. Immunoassays, such as ELISpot, are commonly used to assess the immunogenicity of vaccines towards T cells and B cells. To date, these assays only analyse responses to specific antigens since they are based on recombinant parasite-derived proteins or peptides. There is the need for an agnostic approach that allows the evaluation of all sporozoite-associated antigens.
Methods: ELISpot plates coated with a defined amount of lysed Plasmodium falciparum sporozoites were used to assess the frequency of sporozoite-specific B cells in peripheral blood mononuclear cells from donors immunized with either a recombinant malaria vaccine or irradiated sporozoites.
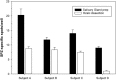
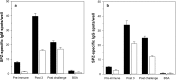
Results: This report describes the assay conditions for a specific and sensitive sporozoite-based B cell ELISpot assay. The assay development considers the quality of sporozoite preparation as well as the detection threshold of the frequency of antigen-specific B cells. The assay enables the detection of sporozoite-specific IgM and IgG-producing B cells. Moreover, the assay can detect sporozoite-reactive B cells from subjects that were either vaccinated with the radiation attenuated sporozoite vaccine or a recombinant pre-erythrocytic vaccine.
Conclusion: The newly developed sporozoite-based B cell ELISpot enables the monitoring of changes in the frequency of sporozoite-specific B cells. Applying this assay to assess the potency of vaccination regimens or seasonal changes in B cell populations from subjects residing in malaria-endemic areas will provide an opportunity to gain insight into immune mechanisms involved in protection and/or disease.
Keywords: Antibodies; B cells; ELISpot; Immunity; Malaria; Sporozoite; Whole parasite vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–771. doi: 10.1093/infdis/jiw237. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

