TRPV1 mediates astrocyte activation and interleukin-1β release induced by hypoxic ischemia (HI)
- PMID: 31142341
- PMCID: PMC6540554
- DOI: 10.1186/s12974-019-1487-3
TRPV1 mediates astrocyte activation and interleukin-1β release induced by hypoxic ischemia (HI)
Abstract
Background: Hypoxic-ischemic encephalopathy (HIE) is a serious birth complication with high incidence in both advanced and developing countries. Children surviving from HIE often have severe long-term sequela including cerebral palsy, epilepsy, and cognitive disabilities. The severity of HIE in infants is tightly associated with increased IL-1β expression and astrocyte activation which was regulated by transient receptor potential vanilloid 1 (TRPV1), a non-selective cation channel in the TRP family.
Methods: Neonatal hypoxic ischemia (HI) and oxygen-glucose deprivation (OGD) were used to simulate HIE in vivo and in vitro. Primarily cultured astrocytes were used for investigating the expression of glial fibrillary acidic protein (GFAP), IL-1β, Janus kinase 2 (JAK2), signal transducer and activator of transcription 3 (STAT3), and activation of the nucleotide-binding, oligomerization domain (NOD)-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome by using Western blot, q-PCR, and immunofluorescence. Brain atrophy, infarct size, and neurobehavioral disorders were evaluated by Nissl staining, 2,3,5-triphenyltetrazolium chloride monohydrate (TTC) staining and neurobehavioral tests (geotaxis reflex, cliff aversion reaction, and grip test) individually.
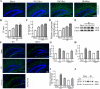
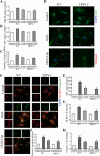
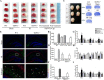
Results: Astrocytes were overactivated after neonatal HI and OGD challenge. The number of activated astrocytes, the expression level of IL-1β, brain atrophy, and shrinking infarct size were all downregulated in TRPV1 KO mice. TRPV1 deficiency in astrocytes attenuated the expression of GFAP and IL-1β by reducing phosphorylation of JAK2 and STAT3. Meanwhile, IL-1β release was significantly reduced in TRPV1 deficiency astrocytes by inhibiting activation of NLRP3 inflammasome. Additionally, neonatal HI-induced neurobehavioral disorders were significantly improved in the TRPV1 KO mice.
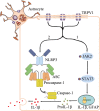
Conclusions: TRPV1 promotes activation of astrocytes and release of astrocyte-derived IL-1β mainly via JAK2-STAT3 signaling and activation of the NLRP3 inflammasome. Our findings provide mechanistic insights into TRPV1-mediated brain damage and neurobehavioral disorders caused by neonatal HI and potentially identify astrocytic TRPV1 as a novel therapeutic target for treating HIE in the subacute stages (24 h).
Keywords: Astrocyte; HI; IL-1β; TRPV1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Confortim Heloísa Deola, Deniz Bruna Ferrary, de Almeida Wellington, Miguel Patrícia Maidana, Bronauth Loise, Vieira Milene Cardoso, de Oliveira Bruna Chaves, Pereira Lenir Orlandi. Neonatal hypoxia-ischemia caused mild motor dysfunction, recovered by acrobatic training, without affecting morphological structures involved in motor control in rats. Brain Research. 2019;1707:27–44. doi: 10.1016/j.brainres.2018.11.021. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

