Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice
- PMID: 31142553
- PMCID: PMC6615698
- DOI: 10.1074/jbc.RA119.007743
Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice
Erratum in
-
Correction: Defining α-synuclein species responsible for Parkinson's disease phenotypes in mice.J Biol Chem. 2020 Jan 24;295(4):1142. doi: 10.1074/jbc.AAC119.012485. J Biol Chem. 2020. PMID: 31980482 Free PMC article. No abstract available.
Abstract
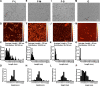
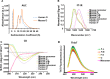
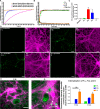
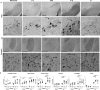
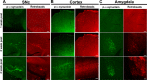
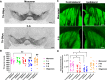
Parkinson's disease (PD) is a neurodegenerative disorder characterized by fibrillar neuronal inclusions composed of aggregated α-synuclein (α-syn). These inclusions are associated with behavioral and pathological PD phenotypes. One strategy for therapeutic interventions is to prevent the formation of these inclusions to halt disease progression. α-Synuclein exists in multiple structural forms, including disordered, nonamyloid oligomers, ordered amyloid oligomers, and fibrils. It is critical to understand which conformers contribute to specific PD phenotypes. Here, we utilized a mouse model to explore the pathological effects of stable β-amyloid-sheet oligomers compared with those of fibrillar α-synuclein. We biophysically characterized these species with transmission EM, atomic-force microscopy, CD spectroscopy, FTIR spectroscopy, analytical ultracentrifugation, and thioflavin T assays. We then injected these different α-synuclein forms into the mouse striatum to determine their ability to induce PD-related phenotypes. We found that β-sheet oligomers produce a small but significant loss of dopamine neurons in the substantia nigra pars compacta (SNc). Injection of small β-sheet fibril fragments, however, produced the most robust phenotypes, including reduction of striatal dopamine terminals, SNc loss of dopamine neurons, and motor-behavior defects. We conclude that although the β-sheet oligomers cause some toxicity, the potent effects of the short fibrillar fragments can be attributed to their ability to recruit monomeric α-synuclein and spread in vivo and hence contribute to the development of PD-like phenotypes. These results suggest that strategies to reduce the formation and propagation of β-sheet fibrillar species could be an important route for therapeutic intervention in PD and related disorders.
Keywords: Lewy body; Parkinson's disease; amyloid; cytotoxicity; fibril; motor-behavior defect; neurodegenerative disease; oligomer; protein aggregation; α-synuclein.
© 2019 Froula et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Volpicelli-Daley L. A., Luk K. C., Patel T. P., Tanik S. A., Riddle D. M., Stieber A., Meaney D. F., Trojanowski J. Q., and Lee V. M. (2011) Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 10.1016/j.neuron.2011.08.033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

