RAB6 and microtubules restrict protein secretion to focal adhesions
- PMID: 31142554
- PMCID: PMC6605799
- DOI: 10.1083/jcb.201805002
RAB6 and microtubules restrict protein secretion to focal adhesions
Abstract
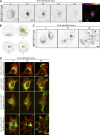
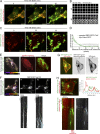
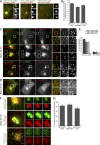
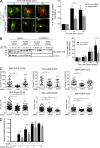
To ensure their homeostasis and sustain differentiated functions, cells continuously transport diverse cargos to various cell compartments and in particular to the cell surface. Secreted proteins are transported along intracellular routes from the endoplasmic reticulum through the Golgi complex before reaching the plasma membrane along microtubule tracks. Using a synchronized secretion assay, we report here that exocytosis does not occur randomly at the cell surface but on localized hotspots juxtaposed to focal adhesions. Although microtubules are involved, the RAB6-dependent machinery plays an essential role. We observed that, irrespective of the transported cargos, most post-Golgi carriers are positive for RAB6 and that its inactivation leads to a broad reduction of protein secretion. RAB6 may thus be a general regulator of post-Golgi secretion.
© 2019 Fourriere et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

