Practical Guidance to Implementing Quality Management Systems in Public Health Laboratories Performing Next-Generation Sequencing: Personnel, Equipment, and Process Management (Phase 1)
- PMID: 31142608
- PMCID: PMC6663893
- DOI: 10.1128/JCM.00261-19
Practical Guidance to Implementing Quality Management Systems in Public Health Laboratories Performing Next-Generation Sequencing: Personnel, Equipment, and Process Management (Phase 1)
Abstract
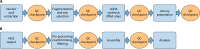
Quality standards as part of an effective quality management system (QMS) are the cornerstone for generating high-quality test results. Next-generation sequencing (NGS) has the potential to improve both clinical diagnostics and public health surveillance efforts in multiple areas, including infectious diseases. However, the laboratories adopting NGS methods face significant challenges due to the complex and modular process design. This document summarizes the first phase of quality system guidance developed by the Centers for Disease Control and Prevention (CDC) NGS Quality Workgroup. The quality system essentials of personnel, equipment, and process management (quality control and validation) were prioritized based on a risk assessment using information gathered from participating CDC laboratories. Here, we present a prioritized QMS framework, including procedures and documentation tools, to assist laboratory implementation and maintenance of quality practices for NGS workflows.
Keywords: next-generation sequencing; public health; quality management systems; quality system essentials.
Figures
References
-
- Kozyreva VK, Truong C-L, Greninger AL, Crandall J, Mukhopadhyay R, Chaturvedi V. 2017. Validation and implementation of Clinical Laboratory Improvements Act-compliant whole-genome sequencing in the public health microbiology laboratory. J Clin Microbiol 55:2502–2520. doi:10.1128/JCM.00361-17. - DOI - PMC - PubMed
-
- Association of Public Health Laboratories. 2016. Next generation sequencing implementation guide. https://www.aphl.org/aboutAPHL/publications/Documents/ID-NGS-Implementat....
-
- Clinical and Laboratory Standards Institute. 2013. The key to quality. CLSI product K2Q Clinical and Laboratory Standards Institute, Wayne, PA.
-
- International Organization for Standardization. 2012. ISO 15189:2012 Medical laboratories—requirements for quality and competence. International Organization for Standardization, Geneva, Switzerland.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources