Anionic Phospholipids Induce Conformational Changes in Phosphoenolpyruvate Carboxylase to Increase Sensitivity to Cathepsin Proteases
- PMID: 31143196
- PMCID: PMC6521631
- DOI: 10.3389/fpls.2019.00582
Anionic Phospholipids Induce Conformational Changes in Phosphoenolpyruvate Carboxylase to Increase Sensitivity to Cathepsin Proteases
Abstract
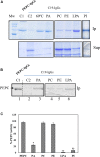
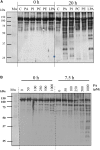
Phosphoenolpyruvate carboxylase (PEPC) is a cytosolic, homotetrameric enzyme that serves a variety of functions in plants, acting as the primary form of CO2 fixation in the C4 photosynthesis pathway (C4-PEPC). In a previous work we have shown that C4-PEPC bind anionic phospholipids, resulting in PEPC inactivation. Also, we showed that PEPC can associate with membranes and to be partially proteolyzed. However, the mechanism controlling this remains unknown. Using semi purified-PEPC from sorghum leaf and a panel of PEPC-specific antibodies, we analyzed the conformational changes in PEPC induced by anionic phospholipids to cause the inactivation of the enzyme. Conformational changes observed involved the exposure of the C-terminus of PEPC from the native, active enzyme conformation. Investigation of the protease activity associated with PEPC demonstrated that cysteine proteases co-purify with the enzyme, with protease-specific substrates revealing cathepsin B and L as the major protease species present. The anionic phospholipid-induced C-terminal exposed conformation of PEPC appeared highly sensitive to the identified cathepsin protease activity and showed initial proteolysis of the enzyme beginning at the N-terminus. Taken together, these data provide the first evidence that anionic phospholipids promote not only the inactivation of the PEPC enzyme, but also its proteolysis.
Keywords: cathepsin proteases; conformational changes; phosphatidic acid; phosphoenolpyruvate carboxylase; phospholipids; proteolysis; sorghum.
Figures




References
-
- Alvarez R., García-Mauriño S., Feria A.-B., Vidal J., Echevarría C. (2003). A conserved 19-amino acid synthetic peptide from the carboxy terminus of phosphoenolpyruvate carboxylase inhibits the in vitro phosphorylation of the enzyme by the calcium-independent phosphoenolpyruvate carboxylase kinase. Plant Physiol. 132 1097–1106. 10.1104/pp.103.023937 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

