Depleting T regulatory cells by targeting intracellular Foxp3 with a TCR mimic antibody
- PMID: 31143508
- PMCID: PMC6527296
- DOI: 10.1080/2162402X.2019.1570778
Depleting T regulatory cells by targeting intracellular Foxp3 with a TCR mimic antibody
Abstract
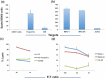
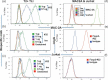
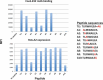
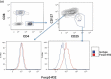
Depletion of T regulatory cells (Tregs) in the tumor microenvironment is a promising cancer immunotherapy strategy. Current approaches for depleting Tregs are limited by lack of specificity and concurrent depletion of anti-tumor effector T cells. The transcription factor forkhead box p3 (Foxp3) plays a central role in the development and function of Tregs and is an ideal target in Tregs, but Foxp3 is an intracellular, undruggable protein to date. We have generated a T cell receptor mimic antibody, "Foxp3-#32," recognizing a Foxp3-derived epitope in the context of HLA-A*02:01. The mAb Foxp3-#32 selectively recognizes CD4 + CD25 + CD127low and Foxp3 + Tregs also expressing HLA-A*02:01 and depletes these cells via antibody-mediated cellular cytotoxicity. Foxp3-#32 mAb depleted Tregs in xenografts of PBMCs from a healthy donor and ascites fluid from a cancer patient. A TCRm mAb targeting intracellular Foxp3 epitope represents an approach to deplete Tregs.
Keywords: Foxp3; Immunosuppression; TCRm mAb; Tregs; immunotherapy.
Figures


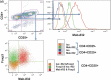

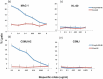




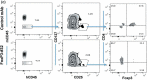


References
-
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B.. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. - PubMed
-
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuur PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
