Adsorption of thallium(I) on rutile nano-titanium dioxide and environmental implications
- PMID: 31143532
- PMCID: PMC6526007
- DOI: 10.7717/peerj.6820
Adsorption of thallium(I) on rutile nano-titanium dioxide and environmental implications
Abstract
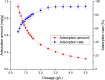
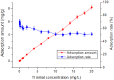
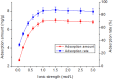
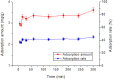
Rutile nano-titanium dioxide (RNTD) characterized by loose particles with diameter in 20-50 nm has a very large surface area for adsorption of Tl, a typical trace metal that has severe toxicity. The increasing application of RNTD and widespread discharge of Tl-bearing effluents from various industrial activities would increase the risk of their co-exposure in aquatic environments. The adsorption behavior of Tl(I) (a prevalent form of Tl in nature) on RNTD was studied as a function of solution pH, temperature, and ion strength. Adsorption isotherms, kinetics, and thermodynamics for Tl(I) were also investigated. The adsorption of Tl(I) on RNTD started at very low pH values and increased abruptly, then maintained at high level with increasing pH >9. Uptake of Tl(I) was very fast on RNTD in the first 15 min then slowed down. The adsorption of Tl(I) on RNTD was an exothermic process; and the adsorption isotherm of Tl(I) followed the Langmuir model, with the maximum adsorption amount of 51.2 mg/g at room temperature. The kinetics of Tl adsorption can be described by a pseudo-second-order equation. FT-IR spectroscopy revealed that -OH and -TiOO-H play an important role in the adsorption. All these results indicate that RNTD has a fast adsorption rate and excellent adsorption amount for Tl(I), which can thus alter the transport, bioavailability and fate of Tl(I) in aqueous environment.
Keywords: Adsorption behavior; Rutile nano-titanium dioxide; Thallium.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


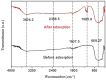

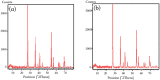
References
-
- Campanella B, Onor M, D’Ulivo A, Giannecchini R, D’Orazio M, Petrini R, Bramanti E. Human exposure to thallium through tap water: a study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy) Science of the Total Environment. 2016;548–549:33–42. doi: 10.1016/j.scitotenv.2016.01.010. - DOI - PubMed
-
- Casiot C, Egal M, Bruneel O, Verma N, Parmentier M, Elbazpoulichet F. Response to comment on predominance of aqueous Tl(I) species in the river system downstream from the Abandoned Carnoulès Mine (Southern France) Environmental Science & Technology. 2011;45:2056–2064. doi: 10.1021/es204479k. - DOI - PubMed
-
- Danielsson K, Persson P, Gallego-Urrea JA, Abbas Z, Rosenqvist J, Jonsson CM. Effects of the adsorption of NOM model molecules on the aggregation of TiO2 nanoparticles in aqueous suspensions. Nanoimpact. 2018;10:177–187. doi: 10.1016/j.impact.2018.05.002. - DOI
LinkOut - more resources
Full Text Sources

