Xpert negative means no TB: A mixed-methods study into early implementation of Xpert in Puducherry, India
- PMID: 31143725
- PMCID: PMC6510069
- DOI: 10.4103/jfmpc.jfmpc_421_18
Xpert negative means no TB: A mixed-methods study into early implementation of Xpert in Puducherry, India
Abstract
Introduction: Xpert MTB/RIF was implemented in 2016 as the initial diagnostic test for extrapulmonary, pediatric, and human immunodeficiency virus-associated tuberculosis (TB) and as an add-on test for sputum microscopy-negative patients under Revised National TB Control Programme, Puducherry, India. We intended to study the change in TB case notification rates (CNRs) after 2015 and explore the enablers and barriers for implementation of Xpert.
Materials and methods: Sequential mixed-methods study, quantitative phase followed by a descriptive qualitative phase (key informant interviews with healthcare providers in the program).
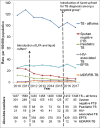
Results: The TB (all forms) CNR increased in 2016 followed by a drop to 2015 levels in 2017. There was a reduction in patients notified as sputum-negative pulmonary TB and pediatric TB during 2016-2017. Healthcare providers used a negative Xpert result in ruling out TB among patients who would previously get diagnosed clinically. Perceived benefits of Xpert were efficiency, rapid results, and detecting resistance. Barriers included poor awareness among medical colleges and the private sector, difficulty in motivating sputum microscopy-negative patients for Xpert, and incompletely filled referral forms.
Conclusion: Xpert-negative results should be interpreted cautiously after clinical assessment. Identified barriers should be addressed to ensure that all eligible undergo testing.
Keywords: Cartridge-based nucleic acid amplification test; India; Structured Operational Research and Training Initiative; initial diagnostic tool for TB; operational research.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- World Health Organization (WHO). Global Tuberculosis Report 2017 [Internet] Geneva, Switzerland: 2017. [Last cited on 2018 Jan 24]. Available from: http://apps.who.int/iris/bitstream/handle/10665/259366/9789241565516-eng... .
-
- Revised national tuberculosis control programme (RNTCP); Central TB division; Ministry of Health and Family Welfare; Government of India. India TB Report 2018 [Internet] New Delhi: 2018. [Last cited on 2018 Jan 24]. Available from: https://tbcindia.gov.in/showfile.php?lid=3314 .
-
- Revised national tuberculosis control programme (RNTCP); Central TB division; Ministry of Health and Family Welfare; Government of India. TB India 2017 [Internet] New Delhi: 2017. [Last cited on 2018 Jan 24]. Available from: http://www.tbcindia.nic.in/index1.php?lang=1&level=1&sublinkid=4737&lid=... .
-
- Kaur R, Kachroo K, Sharma JK, Vatturi SM, Dang A. Diagnostic Accuracy of Xpert Test in Tuberculosis Detection: A Systematic Review and Meta-analysis. [Last cited on 2018 Jan 24];Journal of global infectious diseases [Internet] 8:32–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27013842 . - PMC - PubMed
-
- Detjen AK, DiNardo AR, Leyden J, Steingart KR, Menzies D, Schiller I, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. [Last cited on 2017 Sep 06];The Lancet Respiratory medicine [Internet] 2015 3:451–61. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2213260015000958 . - PMC - PubMed

