Cytocompatible and Anti-bacterial Adhesion Nanotextured Titanium Oxide Layer on Titanium Surfaces for Dental and Orthopedic Implants
- PMID: 31143762
- PMCID: PMC6520600
- DOI: 10.3389/fbioe.2019.00103
Cytocompatible and Anti-bacterial Adhesion Nanotextured Titanium Oxide Layer on Titanium Surfaces for Dental and Orthopedic Implants
Abstract
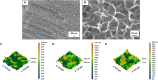
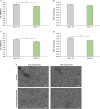
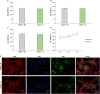
It is widely recognized that surface nanotextures applied on a biomaterial can affect wettability, protein absorption and cellular and/or bacterial adhesion; accordingly, they are nowadays of great interest to promote fast osseointegration and to maintain physiological healing around biomedical implants. In order to be suitable for clinical applications, surface nanotextures must be not only safe and effective, but also, they should be produced through industrial processes scalable to real devices with sustainable processes and costs: this is often a barrier to the market entry. Based on these premises, a chemical surface treatment designed for titanium and its alloys able to produce an oxide layer with a peculiar sponge like nanotexture coupled with high density of hydroxyl group is here presented. The modified Ti-based surfaces previously showed inorganic bioactivity intended as the ability to induce apatite precipitation in simulated body fluid. Physicochemical properties and morphology of the obtained layers have been characterized by means of FESEM, XPS, and Zeta-potential. Biological response to osteoblasts progenitors and bacteria has been tested. The here proposed nanotextured surfaces successfully supported osteoblasts progenitors' adhesion, proliferation and extracellular matrix deposition thus demonstrating good biocompatibility. Moreover, the nanotexture was able to significantly reduce bacteria surface colonization when the orthopedic and the periodontal pathogens Staphylococcus aureus and Aggregatibacter actinomycetemcomitans strains were applied for a short time. Finally, the applicability of the proposed surface treatment to real biomedical devices (a 3D acetabular cup, a dental screw and a micro-sphered laryngeal implant) has been here demonstrated.
Keywords: bacterial adhesion; bone contact; nanotexture; surface modification; titanium.
Figures




References
-
- Bagherifard S., Hickey D. J., de Luca A. C., Malheiro V. N., Markaki A. E., Guagliano M., et al. . (2015). The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 73, 185–197. 10.1016/j.biomaterials.2015.09.019 - DOI - PubMed
-
- Cassinelli C., Morra M., Bruzzone G., Carpi A., Di Santi G., Giardino R., et al. . (2003). Surface chemistry effects of topographic modification of titanium dental implant surfaces: 2. in vitro experiments. Int. J. Oral Maxillofac. Implants 18, 46–52. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

