Immunity in the Cervix: Interphase between Immune and Cervical Epithelial Cells
- PMID: 31143785
- PMCID: PMC6501150
- DOI: 10.1155/2019/7693183
Immunity in the Cervix: Interphase between Immune and Cervical Epithelial Cells
Abstract
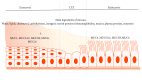
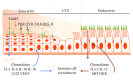
The cervix is divided into two morphologically and immunologically distinct regions, namely, (1) the microbe-laden ectocervix, which is proximal to the vagina, and (2) the "sterile" endocervix, which is distal to the uterus. The two cervical regions are bordered by the cervical transformation zone (CTZ), an area of changing cells, and are predominantly composed of cervical epithelial cells. Epithelial cells are known to play a crucial role in the initiation, maintenance, and regulation of innate and adaptive response in collaboration with immune cells in several tissue types, including the cervix, and their dysfunction can lead to a spectrum of clinical syndromes. For instance, epithelial cells block progression and neutralize or kill microorganisms through multiple ways. These (ways) include mounting physical (intercellular junctions, secretion of mucus) and immune barriers (pathogen-recognition receptor-mediated pathways), which collectively and ultimately lead to the release of specific chemokines and or cytokines. The cytokines subsequently recruit subsets of immune cells appropriate to a particular immune context and response, such as dendritic cells (DCs), T, B, and natural killer (NK) cells. The immune response, as most biological processes in the female reproductive tract (FRT), is mainly regulated by estrogen and progesterone and their (immune cells) responses vary during different physiological phases of reproduction, such as menstrual cycle, pregnancy, and post menopause. The purpose of the present review is to compare the immunological profile of the mucosae and immune cells in the ecto- and endocervix and their interphase during the different phases of female reproduction.
Figures






References
-
- Bekhtereva I. A., Dorosevich A. E. Histophysiology of epithelial and connective tissue components of the vaginal portion of the uterine cervix. Morfologiia. 2009;136(5):90–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

